当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expedited mapping of the ligandable proteome using fully functionalized enantiomeric probe pairs.
Nature Chemistry ( IF 19.2 ) Pub Date : 2019-10-28 , DOI: 10.1038/s41557-019-0351-5 Yujia Wang 1 , Melissa M Dix 1 , Giulia Bianco 2 , Jarrett R Remsberg 1 , Hsin-Yu Lee 1 , Marian Kalocsay 3 , Steven P Gygi 3 , Stefano Forli 2 , Gregory Vite 4 , R Michael Lawrence 4 , Christopher G Parker 1, 5 , Benjamin F Cravatt 1
Nature Chemistry ( IF 19.2 ) Pub Date : 2019-10-28 , DOI: 10.1038/s41557-019-0351-5 Yujia Wang 1 , Melissa M Dix 1 , Giulia Bianco 2 , Jarrett R Remsberg 1 , Hsin-Yu Lee 1 , Marian Kalocsay 3 , Steven P Gygi 3 , Stefano Forli 2 , Gregory Vite 4 , R Michael Lawrence 4 , Christopher G Parker 1, 5 , Benjamin F Cravatt 1
Affiliation
|
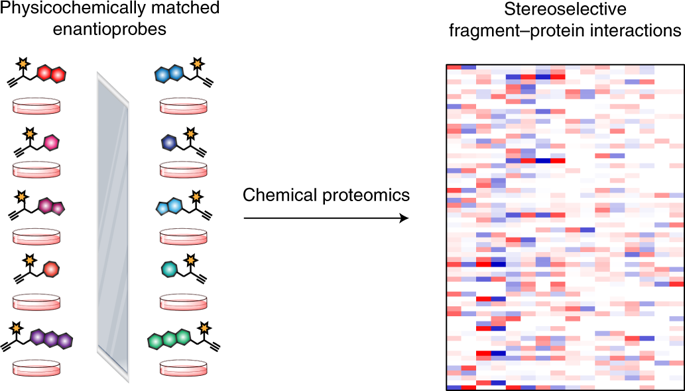
A fundamental challenge in chemical biology and medicine is to understand and expand the fraction of the human proteome that can be targeted by small molecules. We recently described a strategy that integrates fragment-based ligand discovery with chemical proteomics to furnish global portraits of reversible small-molecule/protein interactions in human cells. Excavating clear structure-activity relationships from these 'ligandability' maps, however, was confounded by the distinct physicochemical properties and corresponding overall protein-binding potential of individual fragments. Here, we describe a compelling solution to this problem by introducing a next-generation set of fully functionalized fragments differing only in absolute stereochemistry. Using these enantiomeric probe pairs, or 'enantioprobes', we identify numerous stereoselective protein-fragment interactions in cells and show that these interactions occur at functional sites on proteins from diverse classes. Our findings thus indicate that incorporating chirality into fully functionalized fragment libraries provides a robust and streamlined method to discover ligandable proteins in cells.
中文翻译:

使用完全功能化的对映体探针对快速绘制可配体蛋白质组图谱。
化学生物学和医学的一个基本挑战是了解和扩展可被小分子靶向的人类蛋白质组部分。我们最近描述了一种将基于片段的配体发现与化学蛋白质组学相结合的策略,以提供人类细胞中可逆小分子/蛋白质相互作用的全局肖像。然而,从这些“配位性”图谱中挖掘清晰的结构-活性关系却因各个片段独特的理化特性和相应的整体蛋白质结合潜力而受到混淆。在这里,我们通过引入仅在绝对立体化学方面不同的下一代全功能化片段描述了该问题的一个令人信服的解决方案。使用这些对映体探针对或“对映体探针”,我们鉴定了细胞中的许多立体选择性蛋白质片段相互作用,并表明这些相互作用发生在不同类别蛋白质的功能位点。因此,我们的研究结果表明,将手性纳入功能齐全的片段库中提供了一种稳健且简化的方法来发现细胞中的可配体蛋白质。
更新日期:2019-10-28
中文翻译:

使用完全功能化的对映体探针对快速绘制可配体蛋白质组图谱。
化学生物学和医学的一个基本挑战是了解和扩展可被小分子靶向的人类蛋白质组部分。我们最近描述了一种将基于片段的配体发现与化学蛋白质组学相结合的策略,以提供人类细胞中可逆小分子/蛋白质相互作用的全局肖像。然而,从这些“配位性”图谱中挖掘清晰的结构-活性关系却因各个片段独特的理化特性和相应的整体蛋白质结合潜力而受到混淆。在这里,我们通过引入仅在绝对立体化学方面不同的下一代全功能化片段描述了该问题的一个令人信服的解决方案。使用这些对映体探针对或“对映体探针”,我们鉴定了细胞中的许多立体选择性蛋白质片段相互作用,并表明这些相互作用发生在不同类别蛋白质的功能位点。因此,我们的研究结果表明,将手性纳入功能齐全的片段库中提供了一种稳健且简化的方法来发现细胞中的可配体蛋白质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号