Nature Catalysis ( IF 42.8 ) Pub Date : 2019-10-28 , DOI: 10.1038/s41929-019-0368-6 Haohong Duan , Jin-Cheng Liu , Ming Xu , Yufei Zhao , Xue-Lu Ma , Juncai Dong , Xusheng Zheng , Jianwei Zheng , Christopher S. Allen , Mohsen Danaie , Yung-Kang Peng , Titipong Issariyakul , Dongliang Chen , Angus I. Kirkland , Jean-Charles Buffet , Jun Li , Shik Chi Edman Tsang , Dermot O’Hare
|
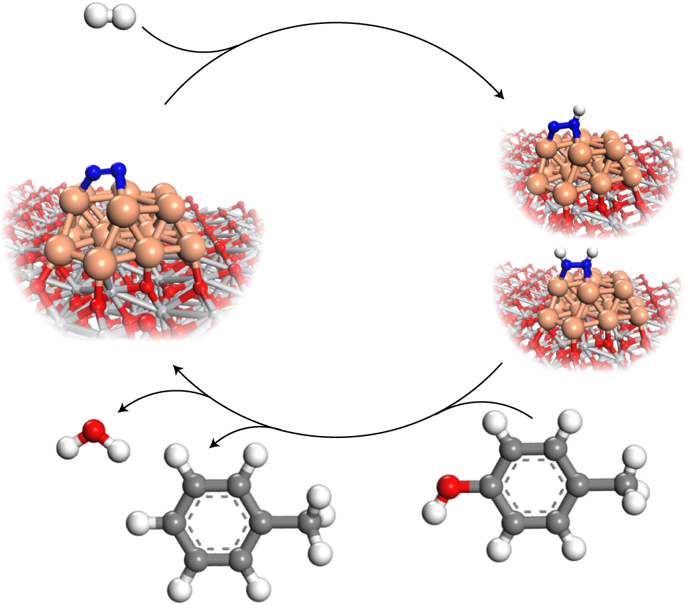
Although molecular dinitrogen (N2) is widely used as a carrier or inert gas for many catalytic reactions, it is rarely considered as a catalytic promoter. Here, we report that N2 could be used to reduce the activation energy for catalytic hydrodeoxygenation over ruthenium-based catalysts. Specifically, we report a 4.3-fold activity increase in the catalytic hydrodeoxygenation of p-cresol to toluene over a titanium oxide supported ruthenium catalyst (Ru/TiO2) by simply introducing 6 bar N2 under batch conditions at 160 °C and 1 bar hydrogen. Detailed investigations indicate that N2 can be adsorbed and activated on the metallic ruthenium surface to form hydrogenated nitrogen species, which offer protic hydrogen to lower the activation energy of direct carbonaromatic–oxygen bond scission and the hydrogenation of hydroxy groups. Thus, by employing different ruthenium catalysts, including Ru/TiO2, Ru/Al2O3, Ru/ZrO2 and Ru/C, we demonstrate that N2 promotion of hydrodeoxygenation can be regarded as a general strategy.
中文翻译:

分子氮促进催化加氢脱氧
尽管分子二氮(N 2)被广泛用作许多催化反应的载体或惰性气体,但很少将其视为催化促进剂。在这里,我们报道N 2可以用来减少钌基催化剂加氢脱氧的活化能。具体而言,我们报告了通过在160°C和1 bar的间歇条件下简单引入6 bar N 2,在氧化钛负载的钌催化剂(Ru / TiO 2)上对甲酚催化加氢脱氧甲苯的活性提高了4.3倍。氢。详细的调查表明,N 2可以被吸附并活化在金属钌表面上,形成氢化的氮,从而提供质子氢,以降低直接碳芳烃-氧键断裂和羟基氢化的活化能。因此,通过使用不同的钌催化剂,包括Ru / TiO 2,Ru / Al 2 O 3,Ru / ZrO 2和Ru / C,我们证明N 2促进加氢脱氧可以被视为一般策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号