Nature Catalysis ( IF 42.8 ) Pub Date : 2019-10-28 , DOI: 10.1038/s41929-019-0370-z Huihui Geng , Xiaobei Chen , Jingjing Gui , Yueteng Zhang , Zuyuan Shen , Pengfei Qian , Junwei Chen , Shilei Zhang , Wei Wang
|
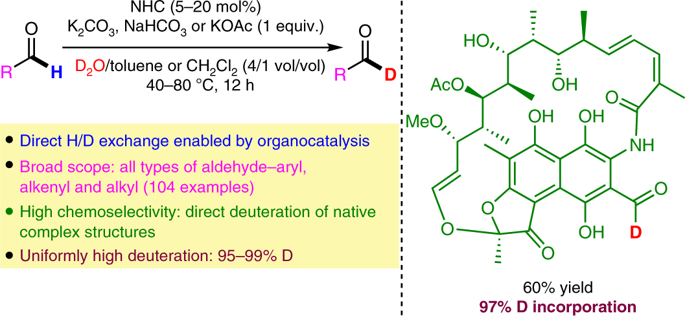
The recent surge in applications of deuterated pharmaceutical agents has created an urgent demand for synthetic methods that efficiently generate deuterated building blocks. Here, we show that N-heterocyclic carbenes promote a reversible hydrogen–deuterium exchange reaction with simple aldehydes, which leads to a practical approach to synthetically valuable C1 deuterated aldehydes. The reactivity of the well-established N-heterocyclic carbene-catalysed formation of Breslow intermediates from aldehydes is reengineered to overcome the overwhelmingly kinetically favourable benzoin condensation reaction and achieve the critical reversibility to drive the formation of desired deuterated products when an excess of D2O is employed. Notably, this operationally simple and cost-effective protocol serves as a general and truly practical approach to all types of 1-D-aldehydes including aryl, alkyl and alkenyl aldehydes, and enables chemoselective late-stage deuterium incorporation into complex, native therapeutic agents and natural products with uniformly high levels (>95%) of deuterium incorporation for a total of 104 tested substrates.
中文翻译:

通过NHC催化实际合成C1氘代醛
近年来,氘代药剂的应用激增,迫切需要有效生成氘代构建基块的合成方法。在这里,我们表明N-杂环卡宾促进了与简单醛的可逆氢-氘交换反应,这导致了合成有价值的C1氘代醛的实用方法。重新设计了由醛形成的公认的N-杂环卡宾催化的Breslow中间体的反应性,以克服绝大多数动力学上有利的安息香缩合反应,并在关键的可逆性下实现了可逆性,从而在过量的D 2时推动了所需的氘代产物的形成使用O。值得注意的是,该操作简单且经济高效的方案可作为所有类型的1-D-醛(包括芳基,烷基和链烯基醛)的通用且真正实用的方法,并且能够将化学选择性的后期氘掺入复杂的天然治疗剂和天然产物,其中氘的掺入量始终保持较高水平(> 95%),共计104种被测底物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号