当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Closer Look at Fe(II) Passivation of Goethite
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-11-11 , DOI: 10.1021/acsearthspacechem.9b00224 Luiza Notini 1 , Drew E. Latta 1 , Anke Neumann 2 , Carolyn I. Pearce 3 , Michel Sassi 3 , Alpha T. N’Diaye 4 , Kevin M. Rosso 3 , Michelle M. Scherer 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-11-11 , DOI: 10.1021/acsearthspacechem.9b00224 Luiza Notini 1 , Drew E. Latta 1 , Anke Neumann 2 , Carolyn I. Pearce 3 , Michel Sassi 3 , Alpha T. N’Diaye 4 , Kevin M. Rosso 3 , Michelle M. Scherer 1
Affiliation
|
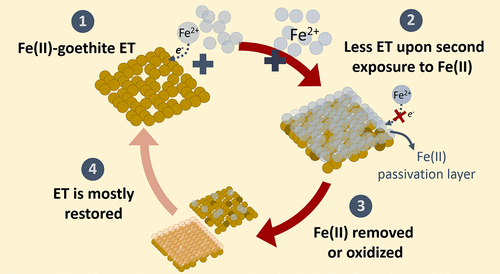
Our understanding of how Fe(II) reacts with Fe(III) oxides has evolved based on evidence for electron transfer at the oxide–water interface and Fe(II)-catalyzed recrystallization. There is, however, some evidence that these, and other processes, such as microbial reduction, cease after continued contact with Fe(II) as the Fe oxide becomes “passivated”. Here, we explore the mechanism of oxide passivation by measuring whether exposure to Fe(II) inhibits Fe(II)–goethite electron transfer, and whether this inhibition is reversible. To quantify the extent of electron transfer, we used selective isotope labeling with 57Fe Mössbauer spectroscopy. We provide experimental evidence that pre-exposure to Fe(II) alters the products formed and inhibits the extent of electron transfer between goethite and Fe(II). We demonstrate that the goethite surface can accumulate a passivation layer of sorbed Fe(II) and that further electron transfer between Fe(II) and goethite is inhibited. Importantly, however, electron transfer can be partially restored upon removal of the layer of Fe(II) by extraction or oxidation. Our results suggest that in environments that are commonly subjected to transient geochemical fluctuations, electron transfer between Fe(II) and Fe oxides, and processes linked to it are likely to be relevant beyond just short time scales.
中文翻译:

针铁矿中的Fe(II)钝化
我们对铁(II)如何与铁(III)氧化物反应的理解是基于在氧化物-水界面的电子转移和铁(II)催化的重结晶的证据而发展的。但是,有一些证据表明,随着Fe氧化物被“钝化”,继续与Fe(II)接触后,这些过程以及其他过程(例如微生物还原)就停止了。在这里,我们通过测量暴露于Fe(II)是否抑制Fe(II)-针铁矿电子转移以及这种抑制作用是否可逆来探讨氧化物钝化的机理。为了量化电子转移的程度,我们使用了选择性同位素标记法(57)FeMössbauer光谱。我们提供的实验证据表明,预先暴露于Fe(II)会改变形成的产物并抑制针铁矿与Fe(II)之间的电子转移程度。我们证明针铁矿表面可以积累吸附的Fe(II)的钝化层,并且进一步抑制了Fe(II)和针铁矿之间的电子转移。然而,重要的是,在通过萃取或氧化去除Fe(II)层后,可以部分恢复电子转移。我们的结果表明,在通常经历瞬态地球化学波动的环境中,Fe(II)和Fe氧化物之间的电子转移以及与之相关的过程很可能在短时间范围内仍具有重要意义。
更新日期:2019-11-11
中文翻译:

针铁矿中的Fe(II)钝化
我们对铁(II)如何与铁(III)氧化物反应的理解是基于在氧化物-水界面的电子转移和铁(II)催化的重结晶的证据而发展的。但是,有一些证据表明,随着Fe氧化物被“钝化”,继续与Fe(II)接触后,这些过程以及其他过程(例如微生物还原)就停止了。在这里,我们通过测量暴露于Fe(II)是否抑制Fe(II)-针铁矿电子转移以及这种抑制作用是否可逆来探讨氧化物钝化的机理。为了量化电子转移的程度,我们使用了选择性同位素标记法(57)FeMössbauer光谱。我们提供的实验证据表明,预先暴露于Fe(II)会改变形成的产物并抑制针铁矿与Fe(II)之间的电子转移程度。我们证明针铁矿表面可以积累吸附的Fe(II)的钝化层,并且进一步抑制了Fe(II)和针铁矿之间的电子转移。然而,重要的是,在通过萃取或氧化去除Fe(II)层后,可以部分恢复电子转移。我们的结果表明,在通常经历瞬态地球化学波动的环境中,Fe(II)和Fe氧化物之间的电子转移以及与之相关的过程很可能在短时间范围内仍具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号