npj Breast Cancer ( IF 6.5 ) Pub Date : 2019-10-28 , DOI: 10.1038/s41523-019-0132-8 Devchand Paul 1, 2 , Svetislava J Vukelja 1, 3 , Frankie Ann Holmes 1, 4 , Joanne L Blum 1, 5 , Kristi J McIntyre 1, 6 , Deborah L Lindquist 1, 7 , Cynthia R Osborne 1, 5 , Ines J Sanchez 1, 8 , Jerome H Goldschmidt 1, 9 , Yunfei Wang 1 , Lina Asmar 1 , Lewis Strauss 10 , Joyce O'Shaughnessy 1, 5
|
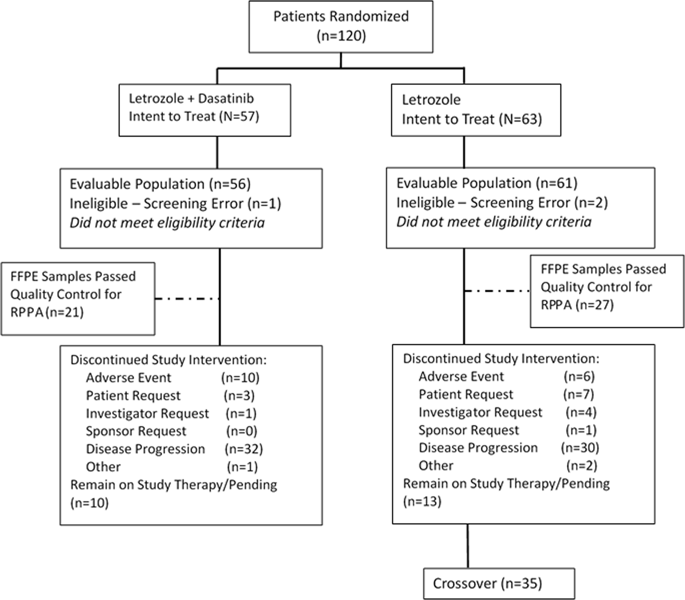
The non-receptor tyrosine kinase Src activation plays a role in the malignant progression of breast cancer, including development of endocrine therapy resistance and survival of bone metastases. This study investigated whether adding Src kinase inhibitor dasatinib to aromatase inhibitor (AI) therapy improved outcomes in estrogen receptor (ER)-positive, HER2-negative metastatic breast cancer (MBC). Postmenopausal patients with ER-positive, HER2-negative MBC (0–1 prior chemotherapies and no prior AI for MBC) were eligible for this non-comparative, parallel group, phase-II study. Patients were randomized to letrozole (2.5 mg/day PO) alone or with dasatinib (100 mg/day PO). Patients with disease progression on letrozole alone could crossover to dasatinib plus continued letrozole. The primary endpoint was clinical-benefit-rate (CBR; complete response + partial response + stable disease ≥6 months). A total of 120 patients were randomized. The CBR of 71% (95% CI 58–83%) was observed with letrozole + dasatinib versus the projected CBR of the combination of 56%. The CBR of 66% (95% CI 52–77%) with letrozole alone also exceeded the projected CBR of 39% with letrozole alone. The CBR was 23% in the crossover arm of letrozole plus dasatinib in patients progressing on letrozole alone. Median progression-free survival with the combination was 20.1 months and 9.9 months with letrozole alone. Letrozole plus dasatinib was well tolerated, although 26% of patients required dasatinib dose reductions. In this non-comparative phase-II trial, the CBR of 71% and the median PFS of 20.1 months with letrozole + dasatinib are encouraging and suggest that dasatinib may inhibit the emergence of acquired resistance to AI therapy.
中文翻译:

来曲唑联合达沙替尼治疗激素受体阳性转移性乳腺癌患者的随机 II 期评估
非受体酪氨酸激酶 Src 激活在乳腺癌的恶性进展中发挥作用,包括内分泌治疗耐药性的发展和骨转移的存活。本研究调查了在芳香酶抑制剂 (AI) 治疗中添加 Src 激酶抑制剂达沙替尼是否可以改善雌激素受体 (ER) 阳性、HER2 阴性转移性乳腺癌 (MBC) 的预后。ER 阳性、HER2 阴性 MBC 的绝经后患者(既往接受过 0-1 次化疗,且既往没有针对 MBC 进行 AI)有资格参加这项非比较、平行组、II 期研究。患者被随机分配单独接受来曲唑(2.5 毫克/天,口服)或与达沙替尼(100 毫克/天,口服)联合治疗。单独使用来曲唑治疗后疾病进展的患者可以交叉使用达沙替尼加继续使用来曲唑。主要终点是临床获益率(CBR;完全缓解+部分缓解+疾病稳定≥6个月)。共有 120 名患者被随机分组。使用来曲唑 + 达沙替尼观察到的 CBR 为 71%(95% CI 58-83%),而预计组合的 CBR 为 56%。单独使用来曲唑的 CBR 为 66%(95% CI 52-77%),也超过了单独使用来曲唑的预计 CBR 39%。在单独使用来曲唑治疗进展的患者中,来曲唑加达沙替尼交叉组的 CBR 为 23%。联合用药的中位无进展生存期为 20.1 个月,而单独使用来曲唑的中位无进展生存期为 9.9 个月。尽管 26% 的患者需要减少达沙替尼剂量,但来曲唑联合达沙替尼的耐受性良好。在这项非比较性 II 期试验中,来曲唑 + 达沙替尼的 CBR 为 71%,中位 PFS 为 20.1 个月,令人鼓舞,表明达沙替尼可能抑制 AI 治疗获得性耐药的出现。











































 京公网安备 11010802027423号
京公网安备 11010802027423号