JAMA Oncology ( IF 22.5 ) Pub Date : 2020-03-01 , DOI: 10.1001/jamaoncol.2019.3692 Zhimin Shao,Da Pang,Hongjian Yang,Wei Li,Shusen Wang,Shude Cui,Ning Liao,Yongsheng Wang,Chuan Wang,Yuan-Ching Chang,Hweichung Wang,Seok Yun Kang,Jae Hong Seo,Kunwei Shen,Suphawat Laohawiriyakamol,Zefei Jiang,Junjie Li,Julian Zhou,Betsy Althaus,Yixiang Mao,Jennifer Eng-Wong
|
Importance Prospective assessment of treatments known to benefit patients in global clinical trials in specific racial groups is essential.
Objective To compare the efficacy, safety, and tolerability of adding pertuzumab to trastuzumab and docetaxel vs placebo, trastuzumab, and docetaxel in Asian patients with ERBB2-positive early or locally advanced breast cancer.
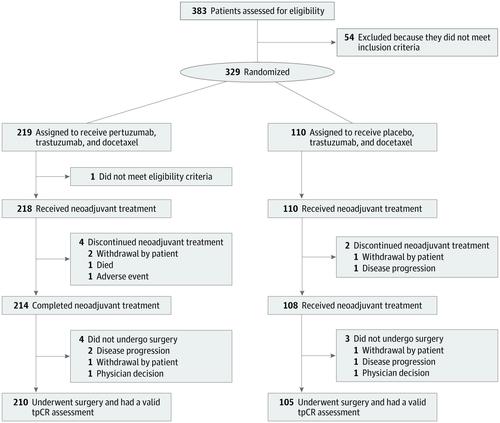
Design, Setting, and Participants This multicenter, double-blind, placebo-controlled phase 3 trial enrolled 329 women with ERBB2-positive early (T2-3, N0-1, M0) or locally advanced breast cancer (T2-3, N2 or N3, M0; T4, any N, M0) and primary tumor larger than 2 cm from March 14, 2016, to March 13, 2017. Analysis of the primary end point was performed on an intention-to-treat basis.
Interventions Before surgery, patients received 4 cycles of intravenous pertuzumab (840-mg loading dose and 420-mg maintenance doses), trastuzumab (8-mg/kg loading dose and 6-mg/kg maintenance doses), and docetaxel (75 mg/m2) or intravenous placebo, trastuzumab, and docetaxel every 3 weeks. After surgery, patients received 3 cycles of intravenous fluorouracil, epirubicin, and cyclophosphamide followed by 13 cycles of the same intravenous anti-ERBB2 therapy (pertuzumab and trastuzumab or placebo and trastuzumab) for up to 1 year.
Main Outcomes and Measures The primary end point was independent review committee–assessed total pathologic complete response rate. The 2-sided Cochran-Mantel-Haenszel test, stratified by disease category and hormone receptor status, was used to compare rates between treatment groups.
Results In total, 329 female patients were randomized (pertuzumab, 219; and placebo, 110; mean [SD] age, 48.8 [9.5] years). In the intention-to-treat population, total pathologic complete response rates were 39.3% (86 of 219) in the pertuzumab group and 21.8% (24 of 110) in the placebo group (difference, 17.5% [95% CI, 6.9%-28.0%]; P = .001). Of the most common grade 3 or higher adverse events, there was a higher incidence of neutropenia in the pertuzumab group (83 of 218 [38.1%] vs 36 of 110 [32.7%]). Serious adverse events were reported in 10.1% of patients (22 of 218) in the pertuzumab group and 8.2% of patients (9 of 110) in the placebo group.
Conclusions and Relevance Treatment with pertuzumab, trastuzumab, and docetaxel resulted in a statistically significant improvement in the total pathologic complete response rate vs placebo, trastuzumab, and docetaxel for the neoadjuvant treatment of ERBB2-positive early or locally advanced breast cancer in Asian patients. Safety data were in line with the known pertuzumab safety profile and generally comparable between treatment groups. The PEONY trial adds to the totality of data showing the benefit of the pertuzumab regimen.
Trial Registration ClinicalTrials.gov identifier: NCT02586025
中文翻译:

培妥珠单抗,曲妥珠单抗和多西他赛对亚洲早期或局部晚期ERBB2阳性乳腺癌患者的疗效,安全性和耐受性:PEONY 3期随机临床试验。
重要性 对在特定种族群体中进行的全球临床试验中有利于患者的治疗进行前瞻性评估至关重要。
目的 比较帕妥珠单抗联合曲妥珠单抗和多西他赛与安慰剂,曲妥珠单抗和多西他赛对亚洲ERBB2阳性早期或局部晚期乳腺癌患者的疗效,安全性和耐受性。
设计,背景和参与者 这项多中心,双盲,安慰剂对照的3期临床试验招募了329例早期ERBB2阳性(T2-3,N0-1,M0)或局部晚期乳腺癌(T2-3,N2或M3)的女性。从2016年3月14日至2017年3月13日,N3,M0; T4,任何N,M0)和大于2 cm的原发肿瘤。主要终点的分析是按意向性治疗进行的。
干预措施 手术前,患者接受了4个周期的静脉曲妥珠单抗(840毫克负荷剂量和420 mg维持剂量),曲妥珠单抗(8 mg / kg负荷剂量和6-mg / kg维持剂量)和多西他赛(75 mg / m 2)或静脉安慰剂,曲妥珠单抗和多西他赛每3周一次。手术后,患者接受3个周期的静脉内氟尿嘧啶,表柔比星和环磷酰胺治疗,然后接受13个周期的相同静脉内抗ERBB2治疗(帕妥珠单抗和曲妥珠单抗或安慰剂和曲妥珠单抗),长达1年。
主要结果和措施 主要终点是独立审查委员会评估的总病理完全缓解率。按疾病类别和激素受体状态进行分层的2面Cochran-Mantel-Haenszel检验用于比较治疗组之间的发生率。
结果 总共有329名女性患者被随机分组(帕妥珠单抗219;安慰剂110;平均[SD]年龄48.8 [9.5]岁)。在意向性治疗人群中,帕妥珠单抗组的总病理学完全缓解率为39.3%(219例中的86例),安慰剂组为21.8%(110例中的24例)(差异为17.5%[95%CI,6.9%] -28.0%];P = 0.001)。在最常见的3级或更高的不良事件中,帕妥珠单抗组中性白细胞减少症的发生率更高(218的83 [38.1%]比110的36 [32.7%])。帕妥珠单抗组中有10.1%的患者(218名中的22名)和安慰剂组中有8.2%的患者(110名中的9名)报告了严重的不良事件。
结论 与帕妥珠单抗,曲妥珠单抗和多西他赛治疗相比,对于亚洲患者中ERBB2阳性的早期或局部晚期乳腺癌的新辅助治疗,与安慰剂,曲妥珠单抗和多西他赛相比,安慰剂,曲妥珠单抗和多西他赛的总病理学完全缓解率具有统计学上的显着改善。安全性数据与已知的帕妥珠单抗安全性相符,并且在治疗组之间通常具有可比性。PEONY试验增加了显示pertuzumab方案获益的全部数据。
试验注册 ClinicalTrials.gov标识符:NCT02586025









































 京公网安备 11010802027423号
京公网安备 11010802027423号