Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2018-09-20 , DOI: 10.1016/j.bbapap.2018.09.004 Stefan Niedermaier , Pitter F. Huesgen
|
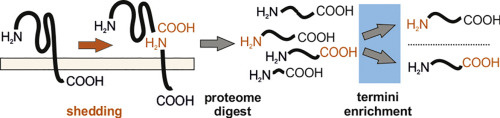
Proteolytic processing shapes cellular interactions with the environment. As a pathway of unconventional protein secretion, ectodomain shedding releases soluble proteoforms of membrane-anchored proteins. This can trigger subsequent cleavage within the membrane stub and the release of additional soluble fragments to intra- and extracellular environments. Distinct membrane-bound proteases, or sheddases, may cleave the same membrane proteins at different sites. Determination of these precise cleavage sites is important, as differently processed proteoforms may exhibit distinct physiological properties and execute antagonistic paracrine and endocrine signaling functions. Conventional quantitative proteomic approaches reliably identify shed proteoforms, but typically not their termini and are thus not able distinguish between functionally different proteoforms differing only by a few amino acids. Dedicated positional proteomics overcomes this challenge and enables proteome-wide identification of protein N- and C-termini. Here, we review positional proteomics techniques, summarize their application to ectodomain shedding and discuss current challenges and developments.
中文翻译:

位置蛋白质组学用于鉴定膜蛋白的定点加工释放的分泌蛋白
蛋白水解加工影响细胞与环境的相互作用。作为非常规蛋白质分泌的途径,胞外域脱落释放出膜锚定蛋白质的可溶性蛋白形式。这可能会触发膜短管内的后续切割,并将其他可溶性片段释放到细胞内和细胞外环境。不同的膜结合蛋白酶或脱氢酶可在不同位点切割相同的膜蛋白。确定这些精确的切割位点很重要,因为不同加工的蛋白形式可能表现出独特的生理特性并执行拮抗旁分泌和内分泌信号传导功能。传统的定量蛋白质组学方法可以可靠地识别脱落的蛋白质形式,但通常不是它们的末端,因此不能区分仅具有几个氨基酸的功能不同的蛋白形式。专用的位置蛋白质组学克服了这一挑战,并能够在蛋白质组范围内鉴定N蛋白和C蛋白。在这里,我们回顾了位置蛋白质组学技术,总结了其在胞外域脱落中的应用,并讨论了当前的挑战和发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号