当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential complementation effects of two disease-associated mutations in tetrameric glutaryl-CoA dehydrogenase is due to inter subunit stability-activity counterbalance.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-09-03 , DOI: 10.1016/j.bbapap.2019.140269 Joana V Ribeiro 1 , Tânia G Lucas 1 , Peter Bross 2 , Cláudio M Gomes 1 , Bárbara J Henriques 1
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-09-03 , DOI: 10.1016/j.bbapap.2019.140269 Joana V Ribeiro 1 , Tânia G Lucas 1 , Peter Bross 2 , Cláudio M Gomes 1 , Bárbara J Henriques 1
Affiliation
|
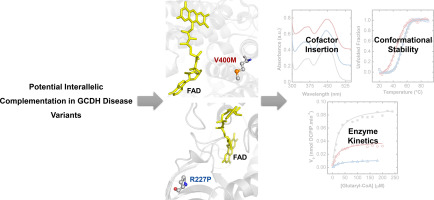
Glutaric Aciduria Type I (GA-I), is an autosomal recessive neurometabolic disease caused by mutations in the GCDH gene that encodes for glutaryl-CoA dehydrogenase (GCDH), a flavoprotein involved in the metabolism of tryptophan, lysine and hydroxylysine. Although over 200 disease mutations have been reported a clear correlation between genotype and phenotype has been difficult to establish. To contribute to a better molecular understanding of GA-I we undertook a detailed molecular study on two GCDH disease-related variants, GCDH-p.Arg227Pro and GCDH-p.Val400Met. Heterozygous patients harbouring these two mutations have increased residual enzymatic activity in relation to homozygous patients with only one of the mutations, suggesting a complementation effect between the two. Combining biochemical, biophysical and structural methods we here establish the effects of these mutations on protein folding, stability and catalytic activity. We show that both variants retain the overall protein fold, but with compromised enzymatic activities. Detailed enzyme kinetic studies reveal that GCDH-p.Arg227Pro has impaired function due to deficient substrate affinity as evidenced by its higher Km, and that the lower activity in GCDH-p.Val400Met results from weaker interactions with its physiological redox partner (electron transfer flavoprotein). Moreover, the GCDH-p.Val400Met variant has a significantly lower thermal stability (ΔTm ≈ 9 °C), and impaired binding of the FAD cofactor in relation to wild-type protein. On these grounds, we provide a rational for the possible interallelic complementation observed in heterozygous patients based on the fact that in GCDH, the low active p.Arg227Pro variant contributes to stabilize the tetramer while the structurally unstable p.Val400Met variant compensates for enzyme activity.
中文翻译:

四聚戊二酰辅酶A脱氢酶中两个与疾病相关的突变的潜在互补效应是由于亚单位间的稳定性-活性平衡所致。
I型戊二酸尿症(GA-I)是一种常染色体隐性遗传性代谢性疾病,由GCDH基因的突变引起,该基因编码戊二酰辅酶A脱氢酶(GCDH),一种参与色氨酸,赖氨酸和羟赖氨酸代谢的黄素蛋白。尽管已经报道了200多种疾病突变,但很难确定基因型和表型之间的明确相关性。为了更好地了解GA-I,我们对两种与GCDH疾病相关的变体GCDH-p.Arg227Pro和GCDH-p.Val400Met进行了详细的分子研究。与仅具有一个突变的纯合患者相比,具有这两个突变的杂合患者具有增加的残留酶活性,这表明两者之间具有互补作用。结合生化,我们在这里通过生物物理和结构方法确定了这些突变对蛋白质折叠,稳定性和催化活性的影响。我们显示这两个变体保留了整体蛋白质折叠,但具有受损的酶活性。详细的酶动力学研究表明,由于底物亲和力不足,GCDH-p.Arg227Pro的功能受损,其高Km证明了这一点,而GCDH-p.Val400Met的较低活性是由于与其生理氧化还原伴侣(电子转移黄素蛋白)的相互作用较弱所致。 )。此外,GCDH-p.Val400Met变体的热稳定性(ΔTm≈9°C)明显较低,并且FAD辅因子与野生型蛋白的结合受损。基于这些理由,
更新日期:2019-10-25
中文翻译:

四聚戊二酰辅酶A脱氢酶中两个与疾病相关的突变的潜在互补效应是由于亚单位间的稳定性-活性平衡所致。
I型戊二酸尿症(GA-I)是一种常染色体隐性遗传性代谢性疾病,由GCDH基因的突变引起,该基因编码戊二酰辅酶A脱氢酶(GCDH),一种参与色氨酸,赖氨酸和羟赖氨酸代谢的黄素蛋白。尽管已经报道了200多种疾病突变,但很难确定基因型和表型之间的明确相关性。为了更好地了解GA-I,我们对两种与GCDH疾病相关的变体GCDH-p.Arg227Pro和GCDH-p.Val400Met进行了详细的分子研究。与仅具有一个突变的纯合患者相比,具有这两个突变的杂合患者具有增加的残留酶活性,这表明两者之间具有互补作用。结合生化,我们在这里通过生物物理和结构方法确定了这些突变对蛋白质折叠,稳定性和催化活性的影响。我们显示这两个变体保留了整体蛋白质折叠,但具有受损的酶活性。详细的酶动力学研究表明,由于底物亲和力不足,GCDH-p.Arg227Pro的功能受损,其高Km证明了这一点,而GCDH-p.Val400Met的较低活性是由于与其生理氧化还原伴侣(电子转移黄素蛋白)的相互作用较弱所致。 )。此外,GCDH-p.Val400Met变体的热稳定性(ΔTm≈9°C)明显较低,并且FAD辅因子与野生型蛋白的结合受损。基于这些理由,











































 京公网安备 11010802027423号
京公网安备 11010802027423号