当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mapping of the binding site for FcμR in human IgM-Fc.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2019-08-23 , DOI: 10.1016/j.bbapap.2019.140266 Rosemary A Nyamboya 1 , Brian J Sutton 1 , Rosaleen A Calvert 1
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 3.2 ) Pub Date : 2019-08-23 , DOI: 10.1016/j.bbapap.2019.140266 Rosemary A Nyamboya 1 , Brian J Sutton 1 , Rosaleen A Calvert 1
Affiliation
|
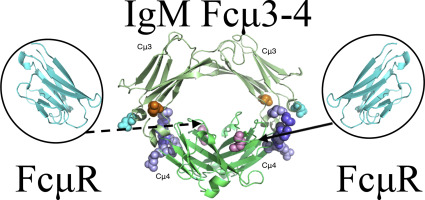
FcμR is a high-affinity receptor for the Fc portion of human IgM. It participates in B cell activation, cell survival and proliferation, but the full range of its functions remains to be elucidated. The receptor has an extracellular immunoglobulin (Ig)-like domain homologous to those in Fcα/μR and pIgR, but unlike these two other IgM receptors which also bind IgA, FcμR exhibits a binding specificity for only IgM-Fc. Previous studies have suggested that the IgM/FcμR interaction mainly involves the Cμ4 domains with possible contributions from either Cμ3 or Cμ2. To define the binding site more precisely, we generated three recombinant IgM-Fc proteins with specific mutations in the Cμ3 and Cμ4 domains, as well as a construct lacking the Cμ2 domains, and analyzed their interaction with the extracellular Ig-like domain of FcμR using surface plasmon resonance analysis. There is a binding site for FcμR in each IgM heavy chain. Neither the absence of the Cμ2 domains nor the quadruple mutant D340S/Q341G/D342S/T343S (in Cμ3 adjacent to Cμ2) affected FcμR binding, whereas double mutant K361D/D416R (in Cμ3 at the Cμ4 interface) substantially decreased binding, and a single mutation Q510R (in Cμ4) completely abolished FcμR binding. We conclude that glutamine at position 510 in Cμ4 is critical for IgM binding to FcμR. This will facilitate discrimination between the distinct effects of FcμR interactions with soluble IgM and with the IgM BCR.
中文翻译:

人IgM-Fc中FcμR结合位点的定位。
FcμR是人IgM Fc部分的高亲和力受体。它参与B细胞活化,细胞存活和增殖,但其全部功能仍有待阐明。该受体具有与Fcα/μR和pIgR同源的细胞外免疫球蛋白(Ig)样结构域,但与这两个也与IgA结合的其他IgM受体不同,FcμR仅对IgM-Fc表现出结合特异性。先前的研究表明,IgM /FcμR相互作用主要涉及Cμ4域,可能来自Cμ3或Cμ2。为了更精确地定义结合位点,我们生成了三个在Cμ3和Cμ4结构域具有特定突变的重组IgM-Fc蛋白,以及一个缺少Cμ2结构域的构建体,并使用表面等离振子共振分析法分析了它们与FcμR胞外Ig样结构域的相互作用。每个IgM重链中都有一个FcμR的结合位点。既不存在Cμ2结构域,也不存在四倍突变体D340S / Q341G / D342S / T343S(在与Cμ2相邻的Cμ3中)影响FcμR结合,而双重突变体K361D / D416R(在Cμ4界面处在Cμ3中)基本上不降低结合力,而单个Q510R突变(在Cμ4中)完全消除了FcμR结合。我们得出的结论是,Cμ4中510位的谷氨酰胺对于IgM与FcμR的结合至关重要。这将有助于区分FcμR与可溶性IgM和IgM BCR相互作用的不同作用。既不存在Cμ2结构域,也不存在四倍突变体D340S / Q341G / D342S / T343S(在与Cμ2相邻的Cμ3中)影响FcμR结合,而双重突变体K361D / D416R(在Cμ4界面处在Cμ3中)基本上没有降低结合。 Q510R突变(在Cμ4中)完全消除了FcμR结合。我们得出的结论是,Cμ4中510位的谷氨酰胺对于IgM与FcμR的结合至关重要。这将有助于区分FcμR与可溶性IgM和IgM BCR相互作用的不同作用。既不存在Cμ2结构域,也不存在四倍突变体D340S / Q341G / D342S / T343S(在与Cμ2相邻的Cμ3中)影响FcμR结合,而双重突变体K361D / D416R(在Cμ4界面处在Cμ3中)基本上不降低结合力,而单个Q510R突变(在Cμ4中)完全消除了FcμR结合。我们得出的结论是,Cμ4中510位的谷氨酰胺对于IgM与FcμR的结合至关重要。这将有助于区分FcμR与可溶性IgM和IgM BCR相互作用的不同作用。
更新日期:2019-10-25
中文翻译:

人IgM-Fc中FcμR结合位点的定位。
FcμR是人IgM Fc部分的高亲和力受体。它参与B细胞活化,细胞存活和增殖,但其全部功能仍有待阐明。该受体具有与Fcα/μR和pIgR同源的细胞外免疫球蛋白(Ig)样结构域,但与这两个也与IgA结合的其他IgM受体不同,FcμR仅对IgM-Fc表现出结合特异性。先前的研究表明,IgM /FcμR相互作用主要涉及Cμ4域,可能来自Cμ3或Cμ2。为了更精确地定义结合位点,我们生成了三个在Cμ3和Cμ4结构域具有特定突变的重组IgM-Fc蛋白,以及一个缺少Cμ2结构域的构建体,并使用表面等离振子共振分析法分析了它们与FcμR胞外Ig样结构域的相互作用。每个IgM重链中都有一个FcμR的结合位点。既不存在Cμ2结构域,也不存在四倍突变体D340S / Q341G / D342S / T343S(在与Cμ2相邻的Cμ3中)影响FcμR结合,而双重突变体K361D / D416R(在Cμ4界面处在Cμ3中)基本上不降低结合力,而单个Q510R突变(在Cμ4中)完全消除了FcμR结合。我们得出的结论是,Cμ4中510位的谷氨酰胺对于IgM与FcμR的结合至关重要。这将有助于区分FcμR与可溶性IgM和IgM BCR相互作用的不同作用。既不存在Cμ2结构域,也不存在四倍突变体D340S / Q341G / D342S / T343S(在与Cμ2相邻的Cμ3中)影响FcμR结合,而双重突变体K361D / D416R(在Cμ4界面处在Cμ3中)基本上没有降低结合。 Q510R突变(在Cμ4中)完全消除了FcμR结合。我们得出的结论是,Cμ4中510位的谷氨酰胺对于IgM与FcμR的结合至关重要。这将有助于区分FcμR与可溶性IgM和IgM BCR相互作用的不同作用。既不存在Cμ2结构域,也不存在四倍突变体D340S / Q341G / D342S / T343S(在与Cμ2相邻的Cμ3中)影响FcμR结合,而双重突变体K361D / D416R(在Cμ4界面处在Cμ3中)基本上不降低结合力,而单个Q510R突变(在Cμ4中)完全消除了FcμR结合。我们得出的结论是,Cμ4中510位的谷氨酰胺对于IgM与FcμR的结合至关重要。这将有助于区分FcμR与可溶性IgM和IgM BCR相互作用的不同作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号