当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Glycosylation effects on the structure and dynamics of a full-length Cel7A cellulase.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-07-04 , DOI: 10.1016/j.bbapap.2019.07.001 Carlos Eduardo Pena 1 , Mauricio G S Costa 2 , Paulo Ricardo Batista 1
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-07-04 , DOI: 10.1016/j.bbapap.2019.07.001 Carlos Eduardo Pena 1 , Mauricio G S Costa 2 , Paulo Ricardo Batista 1
Affiliation
|
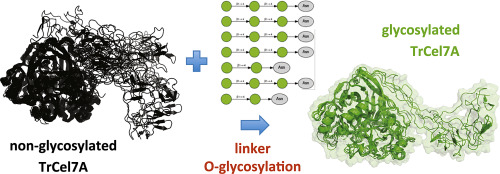
Fungi cellulases are used to degrade cellulose-containing biomass for bioethanol production. Industrial cellulases such as Cel7A from Trichoderma reesei (TrCel7A) are critical in this process. Thus, the understanding of structure and dynamics is crucial for engineering variants with improved cellulolytic activity. This cellulase consists of two domains connected by a flexible and highly glycosylated linker. However, the linker flexibility has hindered the determination of Cel7A complete structure. Herein, based on atomic and sparse data, we applied integrative modelling to build a model of the complete enzyme structure. Next, through simulations, we studied the glycosylation effects on the structure and dynamics of a solubilized TrCel7A. Essential dynamics analysis showed that O-glycosylation in the linker led to the stabilization of protein overall dynamics. O-linked glycans seem to restrict protein dihedral angles distribution in this region, selecting more elongated conformations. Besides the reduced flexibility, functional interdomain motions occurred in a more concerted way in the glycosylated system. In contrast, in the absence of glycosylation, we observed vast conformational plasticity with the functional domains frequently collapsing. We report here evidence that targeting Cel7A linker flexibility by point mutations including modification of glycosylation sites could be a promising design strategy to improve cellulase activity.
中文翻译:

糖基化对全长Cel7A纤维素酶的结构和动力学的影响。
真菌纤维素酶用于降解含纤维素的生物质,用于生产生物乙醇。工业纤维素酶,例如来自里氏木霉的Cel7A(TrCel7A)在此过程中至关重要。因此,对结构和动力学的理解对于具有改善的纤维素分解活性的工程变体是至关重要的。该纤维素酶由通过柔性且高度糖基化的接头连接的两个结构域组成。然而,接头的柔性阻碍了Cel7A完整结构的确定。在此,基于原子和稀疏数据,我们应用了集成建模来构建完整酶结构的模型。接下来,通过模拟,我们研究了糖基化对溶解的TrCel7A的结构和动力学的影响。基本动力学分析表明,接头中的O-糖基化导致蛋白质总体动力学的稳定。O-连接的聚糖似乎限制了该区域中蛋白质二面角的分布,从而选择了更细长的构象。除了降低的灵活性之外,功能性域间运动在糖基化系统中以更协调的方式发生。相反,在没有糖基化的情况下,我们观察到巨大的构象可塑性,其中功能域经常折叠。我们在这里报告的证据表明,通过点突变(包括糖基化位点的修饰)来靶向Cel7A连接子的柔性可能是改善纤维素酶活性的一种有前途的设计策略。除了降低的灵活性之外,功能性域间运动在糖基化系统中以更协调的方式发生。相反,在没有糖基化的情况下,我们观察到巨大的构象可塑性,其中功能域经常折叠。我们在这里报告的证据表明,通过点突变(包括糖基化位点的修饰)来靶向Cel7A连接子的柔性可能是改善纤维素酶活性的有前途的设计策略。除了降低的灵活性之外,功能性域间运动在糖基化系统中以更协调的方式发生。相反,在没有糖基化的情况下,我们观察到巨大的构象可塑性,其中功能域经常折叠。我们在这里报告的证据表明,通过点突变(包括糖基化位点的修饰)来靶向Cel7A连接子的柔性可能是改善纤维素酶活性的一种有前途的设计策略。
更新日期:2019-10-25
中文翻译:

糖基化对全长Cel7A纤维素酶的结构和动力学的影响。
真菌纤维素酶用于降解含纤维素的生物质,用于生产生物乙醇。工业纤维素酶,例如来自里氏木霉的Cel7A(TrCel7A)在此过程中至关重要。因此,对结构和动力学的理解对于具有改善的纤维素分解活性的工程变体是至关重要的。该纤维素酶由通过柔性且高度糖基化的接头连接的两个结构域组成。然而,接头的柔性阻碍了Cel7A完整结构的确定。在此,基于原子和稀疏数据,我们应用了集成建模来构建完整酶结构的模型。接下来,通过模拟,我们研究了糖基化对溶解的TrCel7A的结构和动力学的影响。基本动力学分析表明,接头中的O-糖基化导致蛋白质总体动力学的稳定。O-连接的聚糖似乎限制了该区域中蛋白质二面角的分布,从而选择了更细长的构象。除了降低的灵活性之外,功能性域间运动在糖基化系统中以更协调的方式发生。相反,在没有糖基化的情况下,我们观察到巨大的构象可塑性,其中功能域经常折叠。我们在这里报告的证据表明,通过点突变(包括糖基化位点的修饰)来靶向Cel7A连接子的柔性可能是改善纤维素酶活性的一种有前途的设计策略。除了降低的灵活性之外,功能性域间运动在糖基化系统中以更协调的方式发生。相反,在没有糖基化的情况下,我们观察到巨大的构象可塑性,其中功能域经常折叠。我们在这里报告的证据表明,通过点突变(包括糖基化位点的修饰)来靶向Cel7A连接子的柔性可能是改善纤维素酶活性的有前途的设计策略。除了降低的灵活性之外,功能性域间运动在糖基化系统中以更协调的方式发生。相反,在没有糖基化的情况下,我们观察到巨大的构象可塑性,其中功能域经常折叠。我们在这里报告的证据表明,通过点突变(包括糖基化位点的修饰)来靶向Cel7A连接子的柔性可能是改善纤维素酶活性的一种有前途的设计策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号