The Journal of Supercritical Fluids ( IF 3.4 ) Pub Date : 2019-10-15 , DOI: 10.1016/j.supflu.2019.104661 Guoxing Li , Youjun Lu , Suitao Qi
|
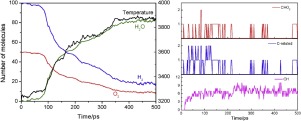
Hydrogen oxidation kinetics in supercritical H2O/CO2 mixtures is a fundamental topic in supercritical water oxidation (SCWO) technology. A series of reactive molecular dynamics (ReaxFF-MD) simulations were performed to investigate hydrogen oxidation process in supercritical mixtures. The results showed that HO2 and H2O2 radicals played key roles in the reaction kinetics. High concentration H2O suppressed the production of OH radical and increased steric hindrance for effective collisions, exerting negative influence on hydrogen oxidation. The presence of CO2 advanced the oxidation rate of hydrogen mainly through the elementary reaction CO2 + H → CO + OH. It was found that high O2 concentration promoted the oxidation of hydrogen and also affected the reaction induction time. The hydrogen reaction mechanisms under supercritical conditions were illustrated, showing different characteristics from those at atmospheric condition. The results will facilitate the development of continuum-scale kinetic models for accurate prediction of hydrogen oxidation behavior in supercritical systems.
中文翻译:

使用ReaxFF分子动力学模拟研究超临界H 2 O / CO 2混合物中的氢氧化
超临界H 2 O / CO 2混合物中的氢氧化动力学是超临界水氧化(SCWO)技术的基本主题。进行了一系列反应分子动力学(ReaxFF-MD)模拟,以研究超临界混合物中的氢氧化过程。结果表明,HO 2和H 2 O 2自由基在反应动力学中起关键作用。高浓度的H 2 O抑制了OH自由基的产生并增加了有效碰撞的空间位阻,对氢氧化产生了负面影响。CO 2的存在主要通过元素反应CO 2来提高氢的氧化速率 + H→CO + OH。发现高浓度的O 2促进了氢的氧化并且还影响了反应的诱导时间。说明了在超临界条件下的氢反应机理,与大气条件下的氢反应机理不同。结果将促进连续尺度动力学模型的发展,以精确预测超临界系统中氢的氧化行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号