Joule ( IF 38.6 ) Pub Date : 2019-10-24 , DOI: 10.1016/j.joule.2019.09.019 Alexander T. Murray , Sahag Voskian , Marcel Schreier , T. Alan Hatton , Yogesh Surendranath
|
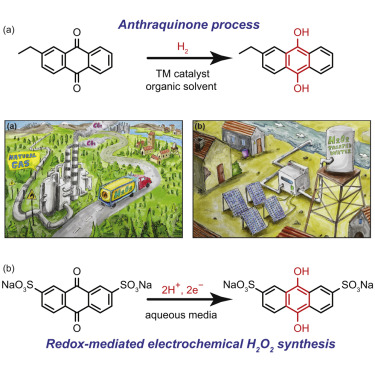
The portable electrochemical generation of hydrogen peroxide (H2O2) from air and water would enable greater utilization of this versatile green oxidant in applications ranging from environmental remediation to portable sanitation. Currently, electrochemical H2O2 synthesis is hampered by the lack of low-cost, non-toxic catalysts that selectively reduce O2 to H2O2 and the lack of low-energy methods for separating the produced H2O2 from the electrolyte media. Herein, we show that a disulfonated anthraquinone can simultaneously catalyze the selective conversion of O2 to H2O2 and shuttle between immiscible aqueous and organic phases via ion exchange. We exploit both of these properties in a flow system to assemble an all-Earth-abundant prototype device for the continuous generation and separation of H2O2 into an electrolyte-free water stream. The combination of molecular redox mediation and phase-transfer catalysis demonstrated here has broad implications for the electrochemical synthesis and isolation of value-added chemicals and fuels.
中文翻译:

相转移催化电合成过氧化氢
由空气和水中的便携式电化学生成过氧化氢(H 2 O 2),将使这种多功能绿色氧化剂在从环境修复到便携式卫生的各种应用中得到更大的利用。目前,电化学ħ 2 ö 2合成由缺乏低成本,无毒性的催化剂的选择性降低Ò阻碍2至H 2 ö 2和用于分离缺少的低能量方法产生的H 2 ö 2从电解质介质。在这里,我们表明,二磺化蒽醌可以同时催化O 2向H的选择性转化。2 O 2并通过离子交换在不混溶的水相和有机相之间穿梭。我们在流动系统中利用这两个特性,组装了一个全地球丰富的原型设备,用于将H 2 O 2连续生成和分离成无电解质的水流。此处证明的分子氧化还原介导和相转移催化的结合对增值化学品和燃料的电化学合成和分离具有广泛的意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号