当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic modeling and mechanistic investigations of transesterification of propylene carbonate with methanol over an Fe–Mn double metal cyanide catalyst
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9re00372j Ziwei Song 1, 2, 3, 4, 5 , Bala Subramaniam 1, 2, 3, 4, 5 , Raghunath V. Chaudhari 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9re00372j Ziwei Song 1, 2, 3, 4, 5 , Bala Subramaniam 1, 2, 3, 4, 5 , Raghunath V. Chaudhari 1, 2, 3, 4, 5
Affiliation
|
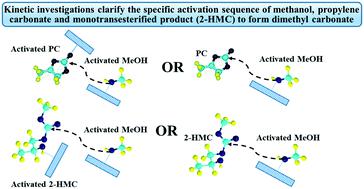
Kinetic modeling of transesterification of propylene carbonate with methanol using an Fe–Mn double metal cyanide catalyst has been investigated based on experimental data obtained under kinetically controlled conditions in a batch slurry reactor in the 140–200 °C range. A simple two-step power law model was found to represent the experimental data well. In addition, a detailed kinetic model based on a molecular level description of the reaction mechanism is also evaluated to provide better insight into the reaction mechanism. It is found that a kinetic model based on the following mechanistic steps provides the best description of the experimental data: (a) activation of methanol by the catalyst to form a methoxy intermediate, (b) activation of propylene carbonate by interaction with the methoxy intermediate to form a second intermediate, (c) reaction of the second intermediate with methoxy species to form a third intermediate which decomposes into the final products dimethyl carbonate and propylene glycol with regeneration of the catalyst precursor. The kinetic model along with mechanistic insights from the present study provides rational guidance for catalyst redesign and process optimization.
中文翻译:

Fe-Mn双金属氰化物催化剂上碳酸亚丙酯与甲醇酯交换反应的动力学模型和机理研究
基于Fe-Mn双金属氰化物催化剂,在动力学控制条件下,在140-200°C的间歇式淤浆反应器中获得的实验数据,研究了碳酸亚丙酯与甲醇的酯交换反应的动力学模型。发现一个简单的两步幂律模型可以很好地表示实验数据。此外,还对基于反应机理的分子水平描述的详细动力学模型进行了评估,以更好地了解反应机理。发现基于以下机理步骤的动力学模型提供了对实验数据的最佳描述:(a)通过催化剂活化甲醇以形成甲氧基中间体,(b)通过与甲氧基中间体相互作用而活化碳酸亚丙酯形成第二种中间体 (c)第二中间体与甲氧基物质反应形成第三中间体,该第三中间体随着催化剂前体的再生而分解成最终产物碳酸二甲酯和丙二醇。动力学模型以及本研究的机理见解为催化剂的重新设计和工艺优化提供了合理的指导。
更新日期:2019-10-24
中文翻译:

Fe-Mn双金属氰化物催化剂上碳酸亚丙酯与甲醇酯交换反应的动力学模型和机理研究
基于Fe-Mn双金属氰化物催化剂,在动力学控制条件下,在140-200°C的间歇式淤浆反应器中获得的实验数据,研究了碳酸亚丙酯与甲醇的酯交换反应的动力学模型。发现一个简单的两步幂律模型可以很好地表示实验数据。此外,还对基于反应机理的分子水平描述的详细动力学模型进行了评估,以更好地了解反应机理。发现基于以下机理步骤的动力学模型提供了对实验数据的最佳描述:(a)通过催化剂活化甲醇以形成甲氧基中间体,(b)通过与甲氧基中间体相互作用而活化碳酸亚丙酯形成第二种中间体 (c)第二中间体与甲氧基物质反应形成第三中间体,该第三中间体随着催化剂前体的再生而分解成最终产物碳酸二甲酯和丙二醇。动力学模型以及本研究的机理见解为催化剂的重新设计和工艺优化提供了合理的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号