当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of menthol from citronellal over supported Ru- and Pt-catalysts in continuous flow
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9re00346k Muhammad Azkaar 1, 2, 3, 4 , Päivi Mäki-Arvela 1, 2, 3, 4 , Zuzana Vajglová 1, 2, 3, 4 , Vyacheslav Fedorov 1, 2, 3, 4, 5 , Narendra Kumar 1, 2, 3, 4 , Leena Hupa 1, 2, 3, 4 , Jarl Hemming 1, 2, 3, 4 , Markus Peurla 1, 2, 3, 4 , Atte Aho 1, 2, 3, 4 , Dmitry Yu Murzin 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9re00346k Muhammad Azkaar 1, 2, 3, 4 , Päivi Mäki-Arvela 1, 2, 3, 4 , Zuzana Vajglová 1, 2, 3, 4 , Vyacheslav Fedorov 1, 2, 3, 4, 5 , Narendra Kumar 1, 2, 3, 4 , Leena Hupa 1, 2, 3, 4 , Jarl Hemming 1, 2, 3, 4 , Markus Peurla 1, 2, 3, 4 , Atte Aho 1, 2, 3, 4 , Dmitry Yu Murzin 1, 2, 3, 4
Affiliation
|
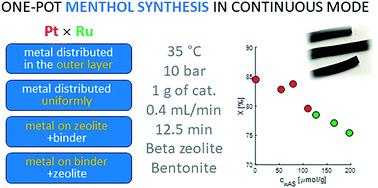
One-pot menthol synthesis from citronellal in a trickle bed reactor was investigated using Ru/H-beta-300 zeolite extrudates without any binder and Pt- and Ru-extrudates containing 70 wt% H-beta-25 zeolites and 30 wt% bentonite binder using different methods of metal loading on the extrudates. The catalytic results were correlated with the physico-chemical properties of bifunctional zeolite-based extrudates. The conversion of citronellal to menthol was nearly the same for Pt extrudates being independent of catalyst acidity or the metal particle size. Ru extrudates also exhibited similar conversion levels as a function of time-on-stream, except for very mildly acidic Ru/beta-300 extrudates, which gave much lower conversion. Pt extrudates showed better stability with time on stream in comparison with Ru extrudates. The best catalyst giving the highest menthol yield and a low amount of acyclic hydrogenation products was Ru-beta-bentonite extrudates where Ru was located randomly in the mixture of 70% H-beta-25 and 30% bentonite binder. Hydrogenation was more prominent for Pt than for Ru catalysts, most probably due to their higher hydrogenation ability. Stereoselectivity to menthol varied in the trickle bed reactor in the range of 67 to 73%. In a batch reactor for Ru- and Pt-catalysts independent of support acidity, it was in the range of 70–71%. The reuse of Ru/H-beta-300 extrudates was successfully demonstrated.
中文翻译:

负载型Ru和Pt催化剂在连续流动条件下从香茅醛中合成薄荷醇
使用不带任何粘合剂的Ru /H-β-300沸石挤出物和含有70 wt%H-beta-25沸石和30 wt%膨润土粘合剂的Pt和Ru挤出物,研究了滴流床反应器中香茅醛一锅合成薄荷醇的情况。在挤出物中使用不同的金属加载方法。催化结果与双功能沸石基挤出物的理化性质相关。对于Pt挤出物而言,香茅醛向薄荷醇的转化几乎相同,而与催化剂的酸度或金属粒度无关。Ru挤出物还显示出类似的转化率,这是随生产时间而变化的,除了极弱酸性的Ru /β-300挤出物,其转化率要低得多。与Ru挤出物相比,Pt挤出物显示出更好的随时间变化的稳定性。给出最高薄荷醇收率和少量无环氢化产物的最佳催化剂是Ru-β-膨润土挤出物,其中Ru随机分布在70%H-β-25和30%膨润土粘合剂的混合物中。Pt的氢化作用比Ru催化剂的氢化作用更为突出,这很可能是因为它们具有更高的氢化能力。在滴流床反应器中,对薄荷醇的立体选择性在67%至73%的范围内变化。在Ru和Pt催化剂的间歇式反应器中,与载体酸度无关,其范围为70-71%。成功证明了Ru / H-beta-300挤出物的再利用。最可能是由于它们更高的氢化能力。在滴流床反应器中,对薄荷醇的立体选择性在67%至73%的范围内变化。在Ru和Pt催化剂的间歇式反应器中,与载体酸度无关,其范围为70-71%。成功证明了Ru / H-beta-300挤出物的再利用。最可能是由于它们更高的氢化能力。在滴流床反应器中,对薄荷醇的立体选择性在67%至73%的范围内变化。在Ru和Pt催化剂的间歇式反应器中,与载体酸度无关,其范围为70-71%。成功证明了Ru / H-beta-300挤出物的再利用。
更新日期:2019-10-24
中文翻译:

负载型Ru和Pt催化剂在连续流动条件下从香茅醛中合成薄荷醇
使用不带任何粘合剂的Ru /H-β-300沸石挤出物和含有70 wt%H-beta-25沸石和30 wt%膨润土粘合剂的Pt和Ru挤出物,研究了滴流床反应器中香茅醛一锅合成薄荷醇的情况。在挤出物中使用不同的金属加载方法。催化结果与双功能沸石基挤出物的理化性质相关。对于Pt挤出物而言,香茅醛向薄荷醇的转化几乎相同,而与催化剂的酸度或金属粒度无关。Ru挤出物还显示出类似的转化率,这是随生产时间而变化的,除了极弱酸性的Ru /β-300挤出物,其转化率要低得多。与Ru挤出物相比,Pt挤出物显示出更好的随时间变化的稳定性。给出最高薄荷醇收率和少量无环氢化产物的最佳催化剂是Ru-β-膨润土挤出物,其中Ru随机分布在70%H-β-25和30%膨润土粘合剂的混合物中。Pt的氢化作用比Ru催化剂的氢化作用更为突出,这很可能是因为它们具有更高的氢化能力。在滴流床反应器中,对薄荷醇的立体选择性在67%至73%的范围内变化。在Ru和Pt催化剂的间歇式反应器中,与载体酸度无关,其范围为70-71%。成功证明了Ru / H-beta-300挤出物的再利用。最可能是由于它们更高的氢化能力。在滴流床反应器中,对薄荷醇的立体选择性在67%至73%的范围内变化。在Ru和Pt催化剂的间歇式反应器中,与载体酸度无关,其范围为70-71%。成功证明了Ru / H-beta-300挤出物的再利用。最可能是由于它们更高的氢化能力。在滴流床反应器中,对薄荷醇的立体选择性在67%至73%的范围内变化。在Ru和Pt催化剂的间歇式反应器中,与载体酸度无关,其范围为70-71%。成功证明了Ru / H-beta-300挤出物的再利用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号