当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Canonical Wnt signaling promotes pacemaker cell specification of cardiac mesodermal cells derived from mouse and human embryonic stem cells
STEM CELLS ( IF 4.0 ) Pub Date : 2019-12-30 , DOI: 10.1002/stem.3106 Wenbin Liang 1 , Pengcheng Han 2 , Elizabeth H Kim 2 , Jordan Mak 2 , Rui Zhang 3 , Angelo G Torrente 3 , Joshua I Goldhaber 3 , Eduardo Marbán 3 , Hee Cheol Cho 2, 4
STEM CELLS ( IF 4.0 ) Pub Date : 2019-12-30 , DOI: 10.1002/stem.3106 Wenbin Liang 1 , Pengcheng Han 2 , Elizabeth H Kim 2 , Jordan Mak 2 , Rui Zhang 3 , Angelo G Torrente 3 , Joshua I Goldhaber 3 , Eduardo Marbán 3 , Hee Cheol Cho 2, 4
Affiliation
|
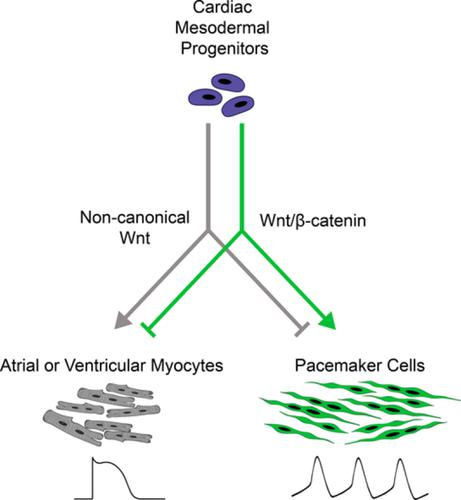
Cardiac differentiation of embryonic stem cells (ESCs) can give rise to de novo chamber cardiomyocytes and nodal pacemaker cells. Compared with our understanding of direct differentiation toward atrial and ventricular myocytes, the mechanisms for nodal pacemaker cell commitment are not well understood. Taking a cue from the prominence of canonical Wnt signaling during cardiac pacemaker tissue development in chick embryos, we asked if modulations of Wnt signaling influence cardiac progenitors to bifurcate to either chamber cardiomyocytes or pacemaker cells. Omitting an exogenous Wnt inhibitor, which is routinely added to maximize cardiac myocyte yield during differentiation of mouse and human ESCs, led to increased yield of spontaneously beating cardiomyocytes with action potential properties similar to those of native sinoatrial node pacemaker cells. The pacemaker phenotype was accompanied by enhanced expression of genes and gene products that mark nodal pacemaker cells such as Hcn4, Tbx18, Tbx3, and Shox2. Addition of exogenous Wnt3a ligand, which activates canonical Wnt/β‐catenin signaling, increased the yield of pacemaker‐like myocytes while reducing cTNT‐positive pan‐cardiac differentiation. Conversely, addition of inhibitors of Wnt/β‐catenin signaling led to increased chamber myocyte lineage development at the expense of pacemaker cell specification. The positive impact of canonical Wnt signaling on nodal pacemaker cell differentiation was evidenced in direct differentiation of two human ESC lines and human induced pluripotent stem cells. Our data identify the Wnt/β‐catenin pathway as a critical determinant of cardiac myocyte subtype commitment during ESC differentiation: endogenous Wnt signaling favors the pacemaker lineage, whereas its suppression promotes the chamber cardiomyocyte lineage.
中文翻译:

经典 Wnt 信号促进源自小鼠和人类胚胎干细胞的心脏中胚层细胞的起搏器细胞规范
胚胎干细胞 (ESC) 的心脏分化可产生新生室心肌细胞和节点起搏器细胞。与我们对心房和心室肌细胞直接分化的理解相比,节点起搏器细胞定型的机制尚不清楚。从鸡胚胎心脏起搏器组织发育过程中经典 Wnt 信号的突出提示,我们询问 Wnt 信号的调节是否会影响心脏祖细胞分叉为腔室心肌细胞或起搏器细胞。省略了外源性 Wnt 抑制剂,在小鼠和人类 ESC 的分化过程中,它通常被添加以最大限度地提高心肌细胞产量,导致自发搏动的心肌细胞的产量增加,其动作电位特性类似于天然窦房结起搏器细胞的动作电位特性。起搏器表型伴随着标记节点起搏器细胞(如 Hcn4、Tbx18、Tbx3 和 Shox2)的基因和基因产物的表达增强。添加外源性 Wnt3a 配体,激活经典 Wnt/β-catenin 信号,增加起搏器样肌细胞的产量,同时减少 cTNT 阳性的泛心脏分化。相反,添加 Wnt/β-catenin 信号抑制剂会导致心室肌细胞谱系发育增加,但会牺牲起搏器细胞的规格。两种人类 ESC 系和人类诱导多能干细胞的直接分化证明了经典 Wnt 信号传导对节点起搏器细胞分化的积极影响。我们的数据确定 Wnt/β-catenin 通路是 ESC 分化过程中心肌细胞亚型定型的关键决定因素:内源性 Wnt 信号有利于起搏器谱系,而其抑制则促进腔室心肌细胞谱系。
更新日期:2019-12-30
中文翻译:

经典 Wnt 信号促进源自小鼠和人类胚胎干细胞的心脏中胚层细胞的起搏器细胞规范
胚胎干细胞 (ESC) 的心脏分化可产生新生室心肌细胞和节点起搏器细胞。与我们对心房和心室肌细胞直接分化的理解相比,节点起搏器细胞定型的机制尚不清楚。从鸡胚胎心脏起搏器组织发育过程中经典 Wnt 信号的突出提示,我们询问 Wnt 信号的调节是否会影响心脏祖细胞分叉为腔室心肌细胞或起搏器细胞。省略了外源性 Wnt 抑制剂,在小鼠和人类 ESC 的分化过程中,它通常被添加以最大限度地提高心肌细胞产量,导致自发搏动的心肌细胞的产量增加,其动作电位特性类似于天然窦房结起搏器细胞的动作电位特性。起搏器表型伴随着标记节点起搏器细胞(如 Hcn4、Tbx18、Tbx3 和 Shox2)的基因和基因产物的表达增强。添加外源性 Wnt3a 配体,激活经典 Wnt/β-catenin 信号,增加起搏器样肌细胞的产量,同时减少 cTNT 阳性的泛心脏分化。相反,添加 Wnt/β-catenin 信号抑制剂会导致心室肌细胞谱系发育增加,但会牺牲起搏器细胞的规格。两种人类 ESC 系和人类诱导多能干细胞的直接分化证明了经典 Wnt 信号传导对节点起搏器细胞分化的积极影响。我们的数据确定 Wnt/β-catenin 通路是 ESC 分化过程中心肌细胞亚型定型的关键决定因素:内源性 Wnt 信号有利于起搏器谱系,而其抑制则促进腔室心肌细胞谱系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号