当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ transformation of a tridentate to a tetradentate unsymmetric Schiff base ligand via deaminative coupling in Ni(II) complexes: crystal structures, magnetic properties and catecholase activity study
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9qi00975b Monotosh Mondal 1, 2, 3, 4, 5 , Soumavo Ghosh 1, 2, 3, 4, 5 , Souvik Maity 1, 2, 3, 4, 5 , Sanjib Giri 1, 5, 6, 7 , Ashutosh Ghosh 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019-10-24 , DOI: 10.1039/c9qi00975b Monotosh Mondal 1, 2, 3, 4, 5 , Soumavo Ghosh 1, 2, 3, 4, 5 , Souvik Maity 1, 2, 3, 4, 5 , Sanjib Giri 1, 5, 6, 7 , Ashutosh Ghosh 1, 2, 3, 4, 5
Affiliation
|
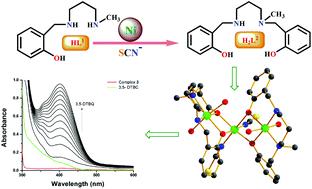
A new dimeric Ni(II) complex, [Ni2L12(CH3CN)4](ClO4)2·2CH3CN (1), was synthesized using an N2O donor reduced Schiff base [(HL1) = 2-[(3-methylamino-propylamino)-methyl]-4-phenol]. Surprisingly, during an attempt to replace its ClO4− ion with SCN−, the N2O donor ligand in situ converted to a tetradentate N2O2 donor ligand and formed a metal complex, [Ni(HL2)(NCS)(CH3CN)] (2). A probable mechanism via deaminative coupling for this conversion is proposed. Using 2 as a metalloligand under basic conditions, a trinuclear metal complex, [Ni3(L2)2(NCS)2(H2O)4]·H2O (3), was prepared. Single crystal structural characterization revealed that in all three metal complexes, the Ni(II) atoms were in an octahedral environment with coordinated solvent molecules (CH3CN in 1 and 2 and H2O in 3). Among the three metal complexes, 1 and 3 showed catecholase-like biomimicking activity. The calculation of the turnover numbers (Kcat = 7.9 for 1, 14.5 for 3) reveals that 3 is a better catalyst than 1. Mechanistic cycles are proposed for this biomimicking activity on the basis of ESI-MS spectrometry and iodometric measurements. Temperature-dependent magnetic susceptibility measurements suggest that the Ni(II) ions in metal complexes 1 and 3 are antiferromagnetically coupled (J = −32.22 cm−1 for 1, J = −10.4 cm−1 for 3), consistent with their geometries and bridging angles. Theoretically calculated J values (J = −40.15 cm−1 for 1, J = −14.53 cm−1 for 3) by the DFT method corroborate well with the experimental values.
中文翻译:

Ni(II)络合物中通过脱氨基偶联从三齿原位转变为四齿不对称席夫碱配体:晶体结构,磁性和儿茶酚酶活性的研究
一种新的二聚的Ni(II)络合物,[倪2大号1 2(CH 3 CN)4 ](CLO 4)2 ·2CH 3 CN(1)中,使用N合成2 ö施主减小的席夫碱[(HL 1)= 2-[((3-甲基氨基-丙基氨基)-甲基] -4-苯酚]。令人惊讶地,企图替代其CLO期间4 -离子与SCN -,N个2 Ó供体配体原位转化为四齿Ñ 2 ö 2供体配体和形成的金属络合物,[镍(HL 2)(NCS)(CH 3 CN)](2)。提出了通过脱氨偶联进行这种转化的可能机理。在碱性条件下使用2作为金属配体,制备了三核金属配合物[Ni 3(L 2)2(NCS)2(H 2 O)4 ]·H 2 O(3)。单晶结构表征表明,在所有三种金属络合物中,Ni(II)原子均处于具有配位溶剂分子的八面体环境中(CH 3 CN位于1和2中,H2 O in 3)。在这三种金属配合物中, 1和3显示出儿茶酚酶样的仿生活性。的转换数的计算( ķ猫= 7.9 1,14.5 3)显示, 3比更好的催化剂1。在ESI-MS光谱法和碘量法测量的基础上,提出了用于这种生物模仿活性的机械循环。温度相关的磁化率测量表明,金属配合物1和3中的Ni( II)离子是反铁磁耦合的( J = -32.22 cm-1( -1), J = -10.4 cm -1( 3),与其几何形状和桥接角度一致。理论计算Ĵ值( Ĵ = -40.15厘米-1为1, Ĵ = -14.53厘米-1为3)通过DFT法确证与实验值。
更新日期:2019-10-24
中文翻译:

Ni(II)络合物中通过脱氨基偶联从三齿原位转变为四齿不对称席夫碱配体:晶体结构,磁性和儿茶酚酶活性的研究
一种新的二聚的Ni(II)络合物,[倪2大号1 2(CH 3 CN)4 ](CLO 4)2 ·2CH 3 CN(1)中,使用N合成2 ö施主减小的席夫碱[(HL 1)= 2-[((3-甲基氨基-丙基氨基)-甲基] -4-苯酚]。令人惊讶地,企图替代其CLO期间4 -离子与SCN -,N个2 Ó供体配体原位转化为四齿Ñ 2 ö 2供体配体和形成的金属络合物,[镍(HL 2)(NCS)(CH 3 CN)](2)。提出了通过脱氨偶联进行这种转化的可能机理。在碱性条件下使用2作为金属配体,制备了三核金属配合物[Ni 3(L 2)2(NCS)2(H 2 O)4 ]·H 2 O(3)。单晶结构表征表明,在所有三种金属络合物中,Ni(II)原子均处于具有配位溶剂分子的八面体环境中(CH 3 CN位于1和2中,H2 O in 3)。在这三种金属配合物中, 1和3显示出儿茶酚酶样的仿生活性。的转换数的计算( ķ猫= 7.9 1,14.5 3)显示, 3比更好的催化剂1。在ESI-MS光谱法和碘量法测量的基础上,提出了用于这种生物模仿活性的机械循环。温度相关的磁化率测量表明,金属配合物1和3中的Ni( II)离子是反铁磁耦合的( J = -32.22 cm-1( -1), J = -10.4 cm -1( 3),与其几何形状和桥接角度一致。理论计算Ĵ值( Ĵ = -40.15厘米-1为1, Ĵ = -14.53厘米-1为3)通过DFT法确证与实验值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号