当前位置:
X-MOL 学术
›
ACS Comb. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Profiling Protein Tyrosine Phosphatase Specificity with Self-Assembled Monolayers for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry and Peptide Arrays.
ACS Combinatorial Science Pub Date : 2019-10-24 , DOI: 10.1021/acscombsci.9b00152 Che-Fan Huang 1 , Milan Mrksich 1, 2
ACS Combinatorial Science Pub Date : 2019-10-24 , DOI: 10.1021/acscombsci.9b00152 Che-Fan Huang 1 , Milan Mrksich 1, 2
Affiliation
|
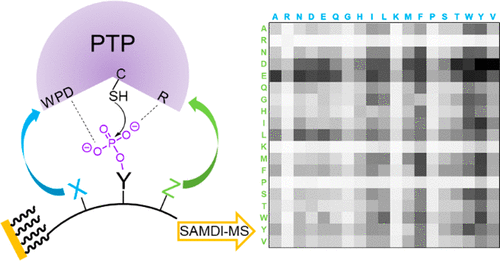
The opposing activities of phosphatases and kinases determine the phosphorylation status of proteins, yet kinases have received disproportionate attention in studies of cellular processes, with the roles of phosphatases remaining less understood. This Research Article describes the use of phosphotyrosine-containing peptide arrays together with matrix-assisted laser desorption/ionization (MALDI) mass spectrometry to directly profile phosphatase substrate selectivities. Twenty-two protein tyrosine phosphatases were characterized with the arrays to give a profile of their specificities. An analysis of the data revealed that certain residues in the substrates had a conserved effect on activity for all enzymes tested, including the general rule that inclusion of a basic lysine or arginine residue on either side of the phosphotyrosine decreased activity. This insight also provides a new perspective on the role of a R1152Q mutant in the insulin receptor, which is known to exhibit a lower phosphorylation level and which this work suggests may be due to an increased activity toward phosphatase enzymes. The use of self-assembled monolayers for matrix-assisted laser desorption/ionization mass spectrometry (SAMDI-MS) to provide a rapid and quantitative assay of phosphatase enzymes will be important to gaining a more complete understanding of the biochemistry and biology of this important enzyme class.
中文翻译:

使用自组装单分子层分析蛋白质酪氨酸磷酸酶特异性,用于基质辅助激光解吸/电离质谱和肽阵列。
磷酸酶和激酶的相反活性决定了蛋白质的磷酸化状态,但激酶在细胞过程研究中受到了过多的关注,而磷酸酶的作用仍知之甚少。本研究文章描述了使用含磷酸酪氨酸的肽阵列和基质辅助激光解吸/电离 (MALDI) 质谱法来直接分析磷酸酶底物选择性。使用芯片对 22 种蛋白质酪氨酸磷酸酶进行了表征,以给出其特异性的概况。对数据的分析表明,底物中的某些残基对所有测试的酶的活性具有保守的影响,包括在磷酸酪氨酸两侧包含碱性赖氨酸或精氨酸残基会降低活性的一般规则。这一见解还为 R1152Q 突变体在胰岛素受体中的作用提供了新的视角,已知胰岛素受体表现出较低的磷酸化水平,这项工作表明这可能是由于对磷酸酶的活性增加所致。使用自组装单分子层进行基质辅助激光解吸/电离质谱 (SAMDI-MS) 来提供磷酸酶的快速定量分析,对于更全面地了解这种重要酶的生物化学和生物学非常重要班级。
更新日期:2019-10-24
中文翻译:

使用自组装单分子层分析蛋白质酪氨酸磷酸酶特异性,用于基质辅助激光解吸/电离质谱和肽阵列。
磷酸酶和激酶的相反活性决定了蛋白质的磷酸化状态,但激酶在细胞过程研究中受到了过多的关注,而磷酸酶的作用仍知之甚少。本研究文章描述了使用含磷酸酪氨酸的肽阵列和基质辅助激光解吸/电离 (MALDI) 质谱法来直接分析磷酸酶底物选择性。使用芯片对 22 种蛋白质酪氨酸磷酸酶进行了表征,以给出其特异性的概况。对数据的分析表明,底物中的某些残基对所有测试的酶的活性具有保守的影响,包括在磷酸酪氨酸两侧包含碱性赖氨酸或精氨酸残基会降低活性的一般规则。这一见解还为 R1152Q 突变体在胰岛素受体中的作用提供了新的视角,已知胰岛素受体表现出较低的磷酸化水平,这项工作表明这可能是由于对磷酸酶的活性增加所致。使用自组装单分子层进行基质辅助激光解吸/电离质谱 (SAMDI-MS) 来提供磷酸酶的快速定量分析,对于更全面地了解这种重要酶的生物化学和生物学非常重要班级。











































 京公网安备 11010802027423号
京公网安备 11010802027423号