当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of Muramic and Glucuronic Acid Glycodendrimers as Dengue Virus Antagonists.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-10-23 , DOI: 10.1002/chem.201903788 Cecilia García-Oliva 1 , Alfredo H Cabanillas 2 , Almudena Perona 1 , Pilar Hoyos 1 , Ángel Rumbero 2 , María J Hernáiz 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-10-23 , DOI: 10.1002/chem.201903788 Cecilia García-Oliva 1 , Alfredo H Cabanillas 2 , Almudena Perona 1 , Pilar Hoyos 1 , Ángel Rumbero 2 , María J Hernáiz 1
Affiliation
|
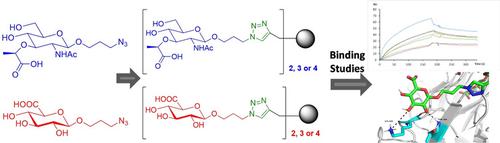
Carbohydrates are involved in many important pathological processes, such as bacterial and viral infections, by means of carbohydrate-protein interactions. Glycoconjugates with multiple carbohydrates are involved in multivalent interactions, thus increasing their binding strengths to proteins. In this work, we report the efficient synthesis of novel muramic and glucuronic acid glycodendrimers as potential Dengue virus antagonists. Aromatic scaffolds functionalized with a terminal ethynyl groups were coupled to muramic and glucuronic acid azides by click chemistry through optimized synthetic strategies to afford the desired glycodendrimers with high yields. Surface Plasmon Resonance studies have demonstrated that the compounds reported bind efficiently to the Dengue virus envelope protein. Molecular modelling studies were carried out to simulate and explain the binding observed. These studies confirm that efficient chemical synthesis of glycodendrimers can be brought about easily offering a versatile strategy to find new active compounds against Dengue virus.
中文翻译:

作为登革热病毒拮抗剂的高效合成的Muramic和葡萄糖醛酸Glycodendrimers。
碳水化合物通过糖-蛋白相互作用参与许多重要的病理过程,例如细菌和病毒感染。具有多种碳水化合物的糖缀合物参与多价相互作用,因此增加了它们与蛋白质的结合强度。在这项工作中,我们报告了新型的山武和葡萄糖醛酸糖树状聚合物作为潜在的登革热病毒拮抗剂的有效合成。通过点击化学,通过优化的合成策略,通过点击化学将具有末端乙炔基官能化的芳族支架偶联至村族和葡糖醛酸叠氮化物,从而以高收率提供所需的糖类树状聚合物。表面等离子体共振研究表明,所报道的化合物与登革热病毒包膜蛋白有效结合。进行了分子建模研究以模拟和解释观察到的结合。这些研究证实,可以轻松地实现糖类树状聚合物的有效化学合成,从而为寻找针对登革热病毒的新活性化合物提供了一种通用的策略。
更新日期:2020-01-24
中文翻译:

作为登革热病毒拮抗剂的高效合成的Muramic和葡萄糖醛酸Glycodendrimers。
碳水化合物通过糖-蛋白相互作用参与许多重要的病理过程,例如细菌和病毒感染。具有多种碳水化合物的糖缀合物参与多价相互作用,因此增加了它们与蛋白质的结合强度。在这项工作中,我们报告了新型的山武和葡萄糖醛酸糖树状聚合物作为潜在的登革热病毒拮抗剂的有效合成。通过点击化学,通过优化的合成策略,通过点击化学将具有末端乙炔基官能化的芳族支架偶联至村族和葡糖醛酸叠氮化物,从而以高收率提供所需的糖类树状聚合物。表面等离子体共振研究表明,所报道的化合物与登革热病毒包膜蛋白有效结合。进行了分子建模研究以模拟和解释观察到的结合。这些研究证实,可以轻松地实现糖类树状聚合物的有效化学合成,从而为寻找针对登革热病毒的新活性化合物提供了一种通用的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号