Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2019-10-22 , DOI: 10.1016/j.comptc.2019.112625 G.A. Poskrebyshev
|
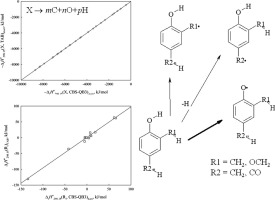
In the present work, the standard thermochemical properties of the most thermochemically stable radicals, produced by abstraction of the hydrogen atom from 2,4-dimethylphenol (2,4-xylenol), 2-methoxy-4-methylphenol, 3-methoxy-4-formylphenol (vanillin), are determined using the results of RO/CBS-4M and RO/CBS-QB3 calculations. It is found that the formation of phenoxy radicals is the most thermochemically favorable pathway for the H atom abstraction from the considered compounds.
The consistent values of the standard enthalpies of formation of the most thermochemically stable structures are determined using the RO/CBS-4M and RO/CBS-QB3 thermochemistry of the homodesmotic reactions, as well as using the correction dependencies.
The values of the standard entropies of these compounds, calculated in the present work, are also reported.
中文翻译:

苯氧基的Δf H o 298.15和S o 298.15的CBS值是通过从替代生物油的成分中提取H原子而形成的
在当前的工作中,通过从2,4-二甲基苯酚(2,4-二甲苯酚),2-甲氧基-4-甲基苯酚,3-甲氧基-4提取氢原子产生的最热化学稳定的自由基的标准热化学性质使用RO / CBS-4M和RO / CBS-QB3计算结果确定-甲酰基苯酚(香兰素)。发现苯氧基的形成是从所考虑的化合物中提取H原子的最热化学上有利的途径。
使用同种脱氢反应的RO / CBS-4M和RO / CBS-QB3热化学以及校正依赖性确定最热化学稳定结构形成的标准焓的一致性值。
还报告了在当前工作中计算出的这些化合物的标准熵的值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号