当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Irreversibility of arsenic trioxide induced PML/RARα fusion protein solubility changes.
Metallomics ( IF 2.9 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9mt00220k Yasen Maimaitiyiming 1 , Yi Ming Shao , Wei Zhong Chen , Yu Jiang , Na Bu , Li Ya Ma , Qian Qian Wang , Xiao Yang Lu , Hua Naranmandura
Metallomics ( IF 2.9 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9mt00220k Yasen Maimaitiyiming 1 , Yi Ming Shao , Wei Zhong Chen , Yu Jiang , Na Bu , Li Ya Ma , Qian Qian Wang , Xiao Yang Lu , Hua Naranmandura
Affiliation
|
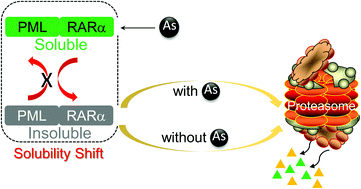
Arsenic trioxide (As2O3) is one of the most effective drugs for the treatment of acute promyelocytic leukemia (APL), and induces the degradation of chimeric oncoprotein PML/RARα (P/R) and APL cell differentiation. Recent evidence has suggested that P/R fusion protein degradation by arsenic occurs through two steps, namely, rapid solubility change/shift of the P/R fusion protein following arsenic treatment (i.e., transfer of P/R protein from the soluble fraction to the insoluble pellet fraction), and subsequent degradation of these insoluble proteins. However, there is little information regarding the reversibility of arsenic induced P/R fusion protein solubility change as well as protein degradation in the insoluble fraction after removing arsenic. In this study, we used APL cell line NB4 or P/R and PML over-expressed 293T cells as well as HeLa cells to reveal the solubility change of P/R and PML by arsenic exposure, and further determined the fate of these insoluble proteins after the removal of arsenic. Here, for the first time, we found that arsenic induced P/R or PML protein solubility change is an irreversible process. Once arsenic induces a P/R or PML protein solubility change, these insoluble proteins could be degraded by the proteasomal pathway even without continuous arsenic treatment. However, PML and P/R proteins can be newly synthesized after the removal of arsenic, suggesting that great caution should be taken in the clinical therapy of APL patients before ending arsenic treatment.
中文翻译:

三氧化二砷诱导的不可逆性PML /RARα融合蛋白的溶解度发生变化。
三氧化二砷(As2O3)是治疗急性早幼粒细胞白血病(APL)的最有效药物之一,并诱导嵌合癌蛋白PML /RARα(P / R)降解和APL细胞分化。最近的证据表明,砷对P / R融合蛋白的降解可通过两个步骤进行,即砷处理后P / R融合蛋白的溶解度快速变化/移位(即P / R蛋白从可溶级分转移至P / R蛋白)。不溶性沉淀部分),以及这些不溶性蛋白的后续降解。但是,关于砷诱导的P / R融合蛋白溶解性变化的可逆性以及去除砷后不溶部分中的蛋白质降解的信息很少。在这项研究中,我们使用APL细胞系NB4或P / R和PML过表达的293T细胞以及HeLa细胞通过砷暴露揭示了P / R和PML的溶解度变化,并进一步确定了这些不溶蛋白在去除砷后的命运。砷。在这里,我们首次发现砷诱导的P / R或PML蛋白溶解度变化是不可逆的过程。砷诱导P / R或PML蛋白溶解度变化后,即使不进行连续砷处理,这些不溶性蛋白也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。并进一步确定了去除砷后这些不溶蛋白的命运。在这里,我们首次发现砷诱导的P / R或PML蛋白溶解度变化是不可逆的过程。砷诱导P / R或PML蛋白溶解度变化后,即使不进行连续砷处理,这些不溶性蛋白也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。并进一步确定了去除砷后这些不溶蛋白的命运。在这里,我们首次发现砷诱导的P / R或PML蛋白溶解度变化是不可逆的过程。砷诱导P / R或PML蛋白溶解度变化后,即使不进行连续砷处理,这些不溶性蛋白也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。即使不进行连续的砷处理,这些不溶性蛋白质也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。即使不进行连续的砷处理,这些不溶性蛋白质也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。
更新日期:2019-12-11
中文翻译:

三氧化二砷诱导的不可逆性PML /RARα融合蛋白的溶解度发生变化。
三氧化二砷(As2O3)是治疗急性早幼粒细胞白血病(APL)的最有效药物之一,并诱导嵌合癌蛋白PML /RARα(P / R)降解和APL细胞分化。最近的证据表明,砷对P / R融合蛋白的降解可通过两个步骤进行,即砷处理后P / R融合蛋白的溶解度快速变化/移位(即P / R蛋白从可溶级分转移至P / R蛋白)。不溶性沉淀部分),以及这些不溶性蛋白的后续降解。但是,关于砷诱导的P / R融合蛋白溶解性变化的可逆性以及去除砷后不溶部分中的蛋白质降解的信息很少。在这项研究中,我们使用APL细胞系NB4或P / R和PML过表达的293T细胞以及HeLa细胞通过砷暴露揭示了P / R和PML的溶解度变化,并进一步确定了这些不溶蛋白在去除砷后的命运。砷。在这里,我们首次发现砷诱导的P / R或PML蛋白溶解度变化是不可逆的过程。砷诱导P / R或PML蛋白溶解度变化后,即使不进行连续砷处理,这些不溶性蛋白也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。并进一步确定了去除砷后这些不溶蛋白的命运。在这里,我们首次发现砷诱导的P / R或PML蛋白溶解度变化是不可逆的过程。砷诱导P / R或PML蛋白溶解度变化后,即使不进行连续砷处理,这些不溶性蛋白也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。并进一步确定了去除砷后这些不溶蛋白的命运。在这里,我们首次发现砷诱导的P / R或PML蛋白溶解度变化是不可逆的过程。砷诱导P / R或PML蛋白溶解度变化后,即使不进行连续砷处理,这些不溶性蛋白也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。即使不进行连续的砷处理,这些不溶性蛋白质也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。即使不进行连续的砷处理,这些不溶性蛋白质也可以通过蛋白酶体途径降解。然而,去除砷后可以重新合成PML和P / R蛋白,这提示在结束砷治疗之前,APL患者的临床治疗应格外小心。











































 京公网安备 11010802027423号
京公网安备 11010802027423号