当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fate of oxygen species from O2 activation at dimetal cofactors in an oxidase enzyme revealed by 57Fe nuclear resonance X-ray scattering and quantum chemistry.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-08-05 , DOI: 10.1016/j.bbabio.2019.148060 Stefan Mebs 1 , Vivek Srinivas 2 , Ramona Kositzki 1 , Julia J Griese 3 , Martin Högbom 2 , Michael Haumann 1
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-08-05 , DOI: 10.1016/j.bbabio.2019.148060 Stefan Mebs 1 , Vivek Srinivas 2 , Ramona Kositzki 1 , Julia J Griese 3 , Martin Högbom 2 , Michael Haumann 1
Affiliation
|
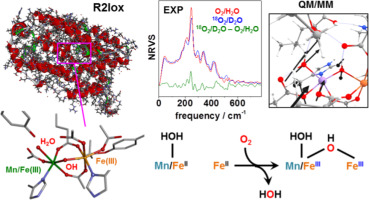
Oxygen (O2) activation is a central challenge in chemistry and catalyzed at prototypic dimetal cofactors in biological enzymes with diverse functions. Analysis of intermediates is required to elucidate the reaction paths of reductive O2 cleavage. An oxidase protein from the bacterium Geobacillus kaustophilus, R2lox, was used for aerobic in-vitro reconstitution with only 57Fe(II) or Mn(II) plus 57Fe(II) ions to yield [FeFe] or [MnFe] cofactors under various oxygen and solvent isotopic conditions including 16/18O and H/D exchange. 57Fe-specific X-ray scattering techniques were employed to collect nuclear forward scattering (NFS) and nuclear resonance vibrational spectroscopy (NRVS) data of the R2lox proteins. NFS revealed Fe/Mn(III)Fe(III) cofactor states and Mössbauer quadrupole splitting energies. Quantum chemical calculations of NRVS spectra assigned molecular structures, vibrational modes, and protonation patterns of the cofactors, featuring a terminal water (H2O) bound at iron or manganese in site 1 and a metal-bridging hydroxide (μOH-) ligand. A procedure for quantitation and correlation of experimental and computational NRVS difference signals due to isotope labeling was developed. This approach revealed that the protons of the ligands as well as the terminal water at the R2lox cofactors exchange with the bulk solvent whereas 18O from 18O2 cleavage is incorporated in the hydroxide bridge. In R2lox, the two water molecules from four-electron O2 reduction are released in a two-step reaction to the solvent. These results establish combined NRVS and QM/MM for tracking of iron-based oxygen activation in biological and chemical catalysts and clarify the reductive O2 cleavage route in an enzyme.
中文翻译:

57Fe核共振X射线散射和量子化学揭示了氧化酶中双金属辅因子处O2活化引起的氧物种的命运。
氧气(O2)的激活是化学领域的主要挑战,并在具有多种功能的生物酶的原型双金属辅因子上得到催化。需要进行中间体分析以阐明还原性O2裂解的反应路径。来自嗜碱芽孢杆菌的氧化酶蛋白R2lox仅用于57Fe(II)或Mn(II)加57Fe(II)离子的有氧体外重组,可在各种氧气和氧气下产生[FeFe]或[MnFe]辅因子。溶剂同位素条件,包括16 / 18O和H / D交换。使用57Fe特异的X射线散射技术收集R2lox蛋白的核前向散射(NFS)和核共振振动光谱(NRVS)数据。NFS揭示了Fe / Mn(III)Fe(III)辅助因子态和Mössbauer四极分裂能。NRVS光谱的量子化学计算分配了辅助因子的分子结构,振动模式和质子化模式,其特征是在位点1处的铁或锰上结合了末端水(H2O)和金属桥连氢氧化物(μOH-)配体。建立了定量和相关性实验和计算NRVS差异信号由于同位素标记的程序。这种方法表明,R2lox辅因子的配体质子以及末端水与大量溶剂交换,而来自18O2裂解的18O被掺入氢氧化物桥中。在R2lox中,来自四电子O2还原的两个水分子以两步反应释放到溶剂中。
更新日期:2019-08-05
中文翻译:

57Fe核共振X射线散射和量子化学揭示了氧化酶中双金属辅因子处O2活化引起的氧物种的命运。
氧气(O2)的激活是化学领域的主要挑战,并在具有多种功能的生物酶的原型双金属辅因子上得到催化。需要进行中间体分析以阐明还原性O2裂解的反应路径。来自嗜碱芽孢杆菌的氧化酶蛋白R2lox仅用于57Fe(II)或Mn(II)加57Fe(II)离子的有氧体外重组,可在各种氧气和氧气下产生[FeFe]或[MnFe]辅因子。溶剂同位素条件,包括16 / 18O和H / D交换。使用57Fe特异的X射线散射技术收集R2lox蛋白的核前向散射(NFS)和核共振振动光谱(NRVS)数据。NFS揭示了Fe / Mn(III)Fe(III)辅助因子态和Mössbauer四极分裂能。NRVS光谱的量子化学计算分配了辅助因子的分子结构,振动模式和质子化模式,其特征是在位点1处的铁或锰上结合了末端水(H2O)和金属桥连氢氧化物(μOH-)配体。建立了定量和相关性实验和计算NRVS差异信号由于同位素标记的程序。这种方法表明,R2lox辅因子的配体质子以及末端水与大量溶剂交换,而来自18O2裂解的18O被掺入氢氧化物桥中。在R2lox中,来自四电子O2还原的两个水分子以两步反应释放到溶剂中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号