当前位置:
X-MOL 学术
›
Comp. Biochem. Physiol. B Biochem. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative analysis of β-hexosaminidase and acid phosphatase from Hydra vulgaris Ind-Pune, H. vulgaris Naukuchiatal and H. magnipapillata sf-1: Localization studies of acid phosphatase and β-hexosaminidase from H. vulgaris Ind-Pune.
Comparative Biochemistry and Physiology B: Biochemistry & Molecular Biology ( IF 1.9 ) Pub Date : 2019-10-17 , DOI: 10.1016/j.cbpb.2019.110365 Rohit Sai Reddy Konada , Lakshmi Surekha Krishnapati , Venugopal Ashapogu , Chung-Hung Lin , Siva Kumar Nadimpalli
Comparative Biochemistry and Physiology B: Biochemistry & Molecular Biology ( IF 1.9 ) Pub Date : 2019-10-17 , DOI: 10.1016/j.cbpb.2019.110365 Rohit Sai Reddy Konada , Lakshmi Surekha Krishnapati , Venugopal Ashapogu , Chung-Hung Lin , Siva Kumar Nadimpalli
|
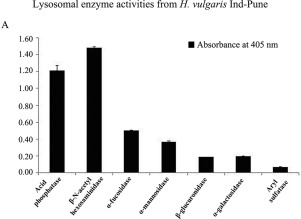
The present report describes a comprehensive study on comparative biochemical characterization of two lysosomal enzymes, acid phosphatase and β-hexosaminidase in three different strains of Hydra; Hydra vulgaris Ind-Pune, H. vulgaris Naukuchiatal and H. magnipapillata sf-1 (self-feeder-1). Since morphology and habitat of Hydra effect lysosomal enzymes and their response to environmental pollutants, it would be interesting to identify them in different Hydra strains so as to use them as toxicity testing. Preliminary studies revealed a differential expression of acid phosphatase, β-hexosaminidase and β-glucuronidase in three Hydra strains. Expression of all three lysosomal enzymes in H. vulgaris Ind-Pune was low in comparison to H. vulgaris Naukuchiatal and H. magnipapillata sf-1, while their expression is comparable in H. vulgaris Naukuchiatal and H. magnipapillata sf-1. The Michaelis-Menten (KM) values for lysosomal β-hexosaminidase using 4-nitrophenyl N-acetyl-β-D-glucosaminide as substrate were found to be 1.3 mM, 1.1 mM and 0.8 mM, respectively for H. vulgaris Ind-Pune, H. vulgaris Naukuchiatal and H. magnipapillata sf-1. For acid phosphatase using 4-nitrophenyl-phosphate as substrate, the KM values were 0.38 mM, 1.2 mM and 0.52 mM respectively, for H. vulgaris Ind-Pune, H. vulgaris Naukuchiatal and sf-1 strains. The optimum temperature for β-hexosaminidase was 60 °C for H. vulgaris Ind-Pune, while 50 °C was observed for H. vulgaris Naukuchiatal and sf-1 strains. The optimum pH for β-hexosaminidase was found to be 6.0 for H. vulgaris Ind-Pune and H. vulgaris Naukuchiatal, and 5.0 for sf-1. The optimum temperature and pH of acid phosphatase was similar in all three strains, viz., 40 °C and 3.0, respectively. Preliminary localization studies using whole mount in situ hybridization revealed predominant endodermal expression of three enzymes in H. vulgaris Ind-Pune. Our results thus support the conservation of lysosomal hydrolases in Hydra.
中文翻译:

九头蛇Ind-Pune,寻常型Naukuchiatal和magnipapillata sf-1的β-己糖胺酶和酸性磷酸酶的比较分析:寻常H. ind.Pune的酸性磷酸酶和β-己糖胺酶的定位研究。
本报告描述了在三种不同的九头蛇菌株中两种溶酶体酶酸性磷酸酶和β-己糖胺酶的比较生化特性的综合研究。九头蛇Ind-Pune,寻常H.Naukuchiatal和H.magnipapillata sf-1(self-feeder-1)。由于九头蛇的形态和生境会影响溶酶体酶及其对环境污染物的响应,因此有必要在不同的九头蛇菌株中鉴定它们,并将其用作毒性测试。初步研究表明,在三种Hydra菌株中酸性磷酸酶,β-己糖胺酶和β-葡萄糖醛酸酶的差异表达。与H. vulgaris Naukuchiatal和H. magnipapillata sf-1相比,H。vulgaris Ind-Pune中所有三种溶酶体酶的表达均较低,而在H. vulgaris Naukuchiatal和H.中它们的表达相当。magnipapillata SF-1。以4-硝基苯基N-乙酰基-β-D-D-氨基葡萄糖为底物的溶酶体β-己糖胺酶的Michaelis-Menten(KM)值分别为H.vulgaris Ind-Pune,1.3 mM,1.1 mM和0.8 mM。 H.寻常型Naukuchiatal和H.magnipapillata sf-1。对于以4-硝基苯基磷酸酯为底物的酸性磷酸酶,寻常型H. vulgaris Ind-Pune,寻常型H. vulgaris Naukuchiatal和sf-1菌株的KM值分别为0.38 mM,1.2 mM和0.52 mM。对于寻常型嗜血杆菌Ind-Pune,β-己糖胺酶的最佳温度为60°C,而对于寻常型嗜血杆菌Naukuchiatal和sf-1菌株,最佳温度为50°C。发现β-己糖胺酶的最佳pH对寻常型Ind-Pune和寻常型Naukuchiatal为6.0,对于sf-1为5.0。在所有三个菌株中,酸性磷酸酶的最佳温度和pH值均相似,即 分别为40°C和3.0。使用整装原位杂交的初步定位研究表明,在寻常型嗜血杆菌Ind-Pune中三种酶的主要内胚层表达。因此,我们的结果支持了在九头蛇中溶酶体水解酶的保存。
更新日期:2019-10-17
中文翻译:

九头蛇Ind-Pune,寻常型Naukuchiatal和magnipapillata sf-1的β-己糖胺酶和酸性磷酸酶的比较分析:寻常H. ind.Pune的酸性磷酸酶和β-己糖胺酶的定位研究。
本报告描述了在三种不同的九头蛇菌株中两种溶酶体酶酸性磷酸酶和β-己糖胺酶的比较生化特性的综合研究。九头蛇Ind-Pune,寻常H.Naukuchiatal和H.magnipapillata sf-1(self-feeder-1)。由于九头蛇的形态和生境会影响溶酶体酶及其对环境污染物的响应,因此有必要在不同的九头蛇菌株中鉴定它们,并将其用作毒性测试。初步研究表明,在三种Hydra菌株中酸性磷酸酶,β-己糖胺酶和β-葡萄糖醛酸酶的差异表达。与H. vulgaris Naukuchiatal和H. magnipapillata sf-1相比,H。vulgaris Ind-Pune中所有三种溶酶体酶的表达均较低,而在H. vulgaris Naukuchiatal和H.中它们的表达相当。magnipapillata SF-1。以4-硝基苯基N-乙酰基-β-D-D-氨基葡萄糖为底物的溶酶体β-己糖胺酶的Michaelis-Menten(KM)值分别为H.vulgaris Ind-Pune,1.3 mM,1.1 mM和0.8 mM。 H.寻常型Naukuchiatal和H.magnipapillata sf-1。对于以4-硝基苯基磷酸酯为底物的酸性磷酸酶,寻常型H. vulgaris Ind-Pune,寻常型H. vulgaris Naukuchiatal和sf-1菌株的KM值分别为0.38 mM,1.2 mM和0.52 mM。对于寻常型嗜血杆菌Ind-Pune,β-己糖胺酶的最佳温度为60°C,而对于寻常型嗜血杆菌Naukuchiatal和sf-1菌株,最佳温度为50°C。发现β-己糖胺酶的最佳pH对寻常型Ind-Pune和寻常型Naukuchiatal为6.0,对于sf-1为5.0。在所有三个菌株中,酸性磷酸酶的最佳温度和pH值均相似,即 分别为40°C和3.0。使用整装原位杂交的初步定位研究表明,在寻常型嗜血杆菌Ind-Pune中三种酶的主要内胚层表达。因此,我们的结果支持了在九头蛇中溶酶体水解酶的保存。











































 京公网安备 11010802027423号
京公网安备 11010802027423号