当前位置:
X-MOL 学术
›
Regul. Toxicol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Subchronic toxicity evaluation of ethanol extract of Cassia tora L. seeds in rats.
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2019-10-01 , DOI: 10.1016/j.yrtph.2019.104487 Mu-Jin Lee 1 , Jong-Hyun Nho 2 , Beo-Deul Yang 2 , Ho Park 2 , Hyun-Joo Lee 2 , Ki-Ho Lee 2 , Ji-Hun Jang 2 , Ho-Kyung Jung 2 , Sun-Ra Kim 2 , Hyun-Woo Cho 2 , Hae-Sung Park 3 , Je-Oh Lim 4 , Jong-Choon Kim 4
Regulatory Toxicology and Pharmacology ( IF 3.0 ) Pub Date : 2019-10-01 , DOI: 10.1016/j.yrtph.2019.104487 Mu-Jin Lee 1 , Jong-Hyun Nho 2 , Beo-Deul Yang 2 , Ho Park 2 , Hyun-Joo Lee 2 , Ki-Ho Lee 2 , Ji-Hun Jang 2 , Ho-Kyung Jung 2 , Sun-Ra Kim 2 , Hyun-Woo Cho 2 , Hae-Sung Park 3 , Je-Oh Lim 4 , Jong-Choon Kim 4
Affiliation
|
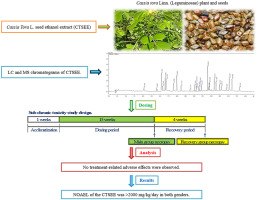
Cassia tora Linn. is an annual or perennial plant of the Fabaceae/Leguminosae family. It is used in traditional medicine for various biological activities including anti-constipation, anti-inflammatory, visual acuity, and hepato-protective activities. The present study was carried out to investigate the potential toxicity of C. tora L. seed ethanol extract (CTSEE) following a 13-week repeated oral administration to Sprague-Dawley rats. CTSEE was administered orally to male and female rats for 13 weeks at 0 (control), 500, 1000, and 2000 mg/kg/day (n = 10, for male and female rats for each dose). Additional recovery groups from the control group and high dose group were observed for a 4-week recovery period. At the end of the treatment and recovery periods, animals were sacrificed, and their organs were weighed and blood samples collected. There were no treatment-related adverse effects in clinical signs, body weight, food consumption, estrous cycle, sperm parameters, urinalysis, hematology, serum biochemistry, necropsy findings, organ weight, and histopathology at any doses tested. Under the present experimental conditions, the no-observed-adverse-effect level of the CTSEE was >2000 mg/kg/day in both genders, and no target organs were identified.
中文翻译:

决明子乙醇提取物对大鼠的亚慢性毒性评价。
决明子tora Linn。是豆科/豆科的一年生或多年生植物。它在传统医学中用于各种生物活动,包括抗便秘,消炎,视力和保护肝脏。进行本研究以调查对Sprague-Dawley大鼠重复口服13周后,C。tora L.种子乙醇提取物(CTSEE)的潜在毒性。将CTSEE分别以0(对照组),500、1000和2000 mg / kg /天的剂量口服给予雄性和雌性大鼠13周(对于每种剂量的雄性和雌性大鼠,n = 10)。观察到对照组和高剂量组的其他恢复组为期4周的恢复期。在治疗和恢复期结束时,处死动物,称其器官称重并收集血样。在任何测试剂量下,临床症状,体重,食物消耗,发情周期,精子参数,尿液分析,血液学,血清生化,尸检结果,器官重量和组织病理学均无治疗相关的不良反应。在目前的实验条件下,两种性别的CTSEE的不良反应水平均未观察到> 2000 mg / kg / day,并且未发现靶器官。
更新日期:2019-10-01
中文翻译:

决明子乙醇提取物对大鼠的亚慢性毒性评价。
决明子tora Linn。是豆科/豆科的一年生或多年生植物。它在传统医学中用于各种生物活动,包括抗便秘,消炎,视力和保护肝脏。进行本研究以调查对Sprague-Dawley大鼠重复口服13周后,C。tora L.种子乙醇提取物(CTSEE)的潜在毒性。将CTSEE分别以0(对照组),500、1000和2000 mg / kg /天的剂量口服给予雄性和雌性大鼠13周(对于每种剂量的雄性和雌性大鼠,n = 10)。观察到对照组和高剂量组的其他恢复组为期4周的恢复期。在治疗和恢复期结束时,处死动物,称其器官称重并收集血样。在任何测试剂量下,临床症状,体重,食物消耗,发情周期,精子参数,尿液分析,血液学,血清生化,尸检结果,器官重量和组织病理学均无治疗相关的不良反应。在目前的实验条件下,两种性别的CTSEE的不良反应水平均未观察到> 2000 mg / kg / day,并且未发现靶器官。











































 京公网安备 11010802027423号
京公网安备 11010802027423号