当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of Vinyl Aziridines with Arynes: Synthesis of Benzazepines and Branched Allyl Fluorides.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-10-19 , DOI: 10.1002/chem.201904727 Sherif J Kaldas 1 , Eva Kran 2 , Christian Mück-Lichtenfeld 2 , Andrei K Yudin 1 , Armido Studer 2
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-10-19 , DOI: 10.1002/chem.201904727 Sherif J Kaldas 1 , Eva Kran 2 , Christian Mück-Lichtenfeld 2 , Andrei K Yudin 1 , Armido Studer 2
Affiliation
|
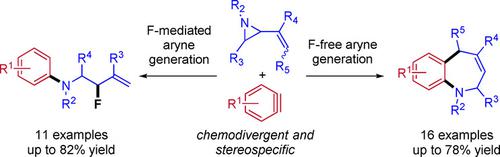
We report the cycloaddition between vinyl aziridines and arynes. Depending on the reaction conditions and the choice of the aryne precursor, the aziridinium intermediate can be trapped through two distinct mechanistic pathways. The first one proceeds through a formal [5+2] cycloaddition to furnish valuable multi-substituted benzazepines. In the second pathway, the aziridinium is intercepted by a fluoride ion to afford allylic fluorides in good yields. Both reactions proceed stereospecifically and furnish enantiopure benzazepines and allylic fluorides.
中文翻译:

乙烯基氮丙啶与芳烃的反应:苯并ze庚因和支链烯丙基氟化物的合成。
我们报告乙烯基氮丙啶和芳烃之间的环加成反应。根据反应条件和芳烃前体的选择,叠氮基中间体可以通过两种不同的机理途径被捕获。第一个过程通过正式的[5 + 2]环加成反应进行,以提供有价值的多取代苯并ze庚因。在第二种途径中,叠氮鎓被氟离子拦截,以高收率得到烯丙基氟化物。两种反应均立体定向进行,并提供对映纯的苯并ze庚因和烯丙基氟化物。
更新日期:2020-01-23
中文翻译:

乙烯基氮丙啶与芳烃的反应:苯并ze庚因和支链烯丙基氟化物的合成。
我们报告乙烯基氮丙啶和芳烃之间的环加成反应。根据反应条件和芳烃前体的选择,叠氮基中间体可以通过两种不同的机理途径被捕获。第一个过程通过正式的[5 + 2]环加成反应进行,以提供有价值的多取代苯并ze庚因。在第二种途径中,叠氮鎓被氟离子拦截,以高收率得到烯丙基氟化物。两种反应均立体定向进行,并提供对映纯的苯并ze庚因和烯丙基氟化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号