当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Calcination kinetics of cement raw meals under various CO2 concentrations
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2019-10-18 , DOI: 10.1039/c9re00361d Jose Ramon Fernandez 1, 2, 3, 4 , Sandra Turrado 1, 2, 3, 4 , Juan Carlos Abanades 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2019-10-18 , DOI: 10.1039/c9re00361d Jose Ramon Fernandez 1, 2, 3, 4 , Sandra Turrado 1, 2, 3, 4 , Juan Carlos Abanades 1, 2, 3, 4
Affiliation
|
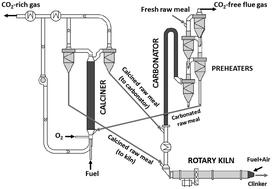
The calcium looping CO2 capture process, CaL, represents a promising option for the decarbonisation of cement plants, due to the intrinsic benefit of using the spent CO2 sorbent as a feedstock for the plant. The generation of sufficiently active CaO from the raw meals entering the cement plant for the CO2 capture requires calcination of these materials at around 900 °C in various atmospheres of CO2. This work investigates the calcination kinetics of fine particles (<50 μm) of limestone, natural marls and raw meals in a drop tube reactor, under conditions very similar to those expected in suspension calciners of CaL systems. Experiments have been carried out with very short gas–solid contact times (t < 2 s) and various concentrations of CO2 (up to 85 vol%). High calcination conversions have been measured under these conditions with all the materials tested regardless of their origin and composition. The kinetic rates of CaCO3 decomposition depend on the BET surface area of the solid, which is consistent with the model reported by Borgwardt, AIChE J., 1985, 31, 103–111, and yield consistent activation energy (i.e. 195 kJ mol−1) and pre-exponential factors when using a dependency on CO2, as proposed by J. M. Valverde, P. E. Sanchez-Jimenez and L. A. Perez-Maqueda, J. Phys. Chem. C, 2015, 119, 1623–1641.
中文翻译:

各种CO2浓度下水泥生料的煅烧动力学
钙循环式CO 2捕集工艺CaL代表了水泥厂脱碳的一个有前途的选择,因为使用废CO 2吸附剂作为工厂的原料具有内在的好处。从进入水泥厂的生料中产生足够活性的CaO来捕获CO 2,需要在900℃左右的各种CO 2气氛下对这些材料进行煅烧。这项工作研究了在滴管反应器中,在非常类似于CaL系统悬浮煅烧炉中预期的条件下,石灰石,天然泥灰和生料的细颗粒(<50μm)的煅烧动力学。实验是在很短的气固接触时间(t<2 s)和各种浓度的CO 2(最高85 vol%)。在这些条件下,已测试所有材料的高煅烧转化率,而不论其来源和组成如何。碳酸钙的动力学速率3分解取决于固体的BET表面积,这是与由博格瓦特,报道的模型一致AIChE的J.,1985,31,103-111,和产生一致的活化能(即195千焦耳摩尔- 1)以及使用对CO 2的依赖关系时的指数前因子,如JM Valverde,PE Sanchez-Jimenez和LA Perez-Maqueda,J. Phys。化学 Ç,2015,119,1623年至1641年。
更新日期:2019-10-18
中文翻译:

各种CO2浓度下水泥生料的煅烧动力学
钙循环式CO 2捕集工艺CaL代表了水泥厂脱碳的一个有前途的选择,因为使用废CO 2吸附剂作为工厂的原料具有内在的好处。从进入水泥厂的生料中产生足够活性的CaO来捕获CO 2,需要在900℃左右的各种CO 2气氛下对这些材料进行煅烧。这项工作研究了在滴管反应器中,在非常类似于CaL系统悬浮煅烧炉中预期的条件下,石灰石,天然泥灰和生料的细颗粒(<50μm)的煅烧动力学。实验是在很短的气固接触时间(t<2 s)和各种浓度的CO 2(最高85 vol%)。在这些条件下,已测试所有材料的高煅烧转化率,而不论其来源和组成如何。碳酸钙的动力学速率3分解取决于固体的BET表面积,这是与由博格瓦特,报道的模型一致AIChE的J.,1985,31,103-111,和产生一致的活化能(即195千焦耳摩尔- 1)以及使用对CO 2的依赖关系时的指数前因子,如JM Valverde,PE Sanchez-Jimenez和LA Perez-Maqueda,J. Phys。化学 Ç,2015,119,1623年至1641年。



























 京公网安备 11010802027423号
京公网安备 11010802027423号