当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrostatically Driven Guanidinium Interaction Domains that Control Hydrogel-Mediated Protein Delivery In Vivo
ACS Central Science ( IF 12.7 ) Pub Date : 2019-10-18 , DOI: 10.1021/acscentsci.9b00501 Stephen E Miller 1 , Yuji Yamada 1 , Nimit Patel 2 , Ernesto Suárez 2 , Caroline Andrews 1 , Steven Tau 1 , Brian T Luke 2 , Raul E Cachau 2 , Joel P Schneider 1
ACS Central Science ( IF 12.7 ) Pub Date : 2019-10-18 , DOI: 10.1021/acscentsci.9b00501 Stephen E Miller 1 , Yuji Yamada 1 , Nimit Patel 2 , Ernesto Suárez 2 , Caroline Andrews 1 , Steven Tau 1 , Brian T Luke 2 , Raul E Cachau 2 , Joel P Schneider 1
Affiliation
|
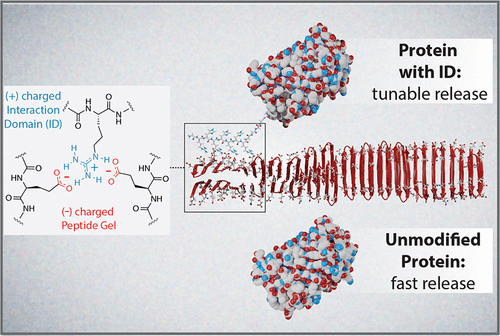
Protein biologics are an important class of drugs, but the necessity for frequent parenteral administration is a major limitation. Drug-delivery materials offer a potential solution, but protein-material adsorption can cause denaturation, which reduces their effectiveness. Here, we describe a new protein delivery platform that limits direct contact between globular protein domains and material matrix, yet from a single subcutaneous administration can be tuned for long-term drug release. The strategy utilizes complementary electrostatic interactions made between a suite of designed interaction domains (IDs), installed onto the terminus of a protein of interest, and a negatively charged self-assembled fibrillar hydrogel. These intermolecular interactions can be easily modulated by choice of ID to control material interaction and desorption energies, which allows regulation of protein release kinetics to fit desired release profiles. Molecular dynamics studies provided a molecular-level understanding of the mechanisms that govern release and identified optimal binding zones on the gel fibrils that facilitate strong ID–material interactions, which are crucial for sustained release of protein. This delivery platform can be easily loaded with cargo, is shear-thin syringe implantable, provides improved protein stability, is capable of a diverse range of in vitro release rates, and most importantly, can accomplish long-term control over in vivo protein delivery.
中文翻译:

静电驱动的胍相互作用域控制水凝胶介导的体内蛋白质递送
蛋白质生物制剂是一类重要的药物,但频繁肠外给药的必要性是一个主要限制。药物输送材料提供了一种潜在的解决方案,但蛋白质材料吸附会导致变性,从而降低其有效性。在这里,我们描述了一种新的蛋白质递送平台,该平台限制了球状蛋白质结构域和材料基质之间的直接接触,但可以通过单次皮下注射进行调整以实现长期药物释放。该策略利用了一组设计的相互作用域(ID)和带负电的自组装纤维水凝胶之间的互补静电相互作用,这些相互作用域安装在感兴趣的蛋白质的末端。这些分子间相互作用可以通过选择 ID 来轻松调节,以控制材料相互作用和解吸能量,从而可以调节蛋白质释放动力学以适应所需的释放曲线。分子动力学研究提供了对控制释放机制的分子水平理解,并确定了凝胶原纤维上的最佳结合区域,这些区域促进了强ID-材料相互作用,这对于蛋白质的持续释放至关重要。该递送平台可以轻松装载货物,可植入剪切薄注射器,提供改进的蛋白质稳定性,能够实现多种体外释放速率,最重要的是,可以实现对体内蛋白质递送的长期控制。
更新日期:2019-11-28
中文翻译:

静电驱动的胍相互作用域控制水凝胶介导的体内蛋白质递送
蛋白质生物制剂是一类重要的药物,但频繁肠外给药的必要性是一个主要限制。药物输送材料提供了一种潜在的解决方案,但蛋白质材料吸附会导致变性,从而降低其有效性。在这里,我们描述了一种新的蛋白质递送平台,该平台限制了球状蛋白质结构域和材料基质之间的直接接触,但可以通过单次皮下注射进行调整以实现长期药物释放。该策略利用了一组设计的相互作用域(ID)和带负电的自组装纤维水凝胶之间的互补静电相互作用,这些相互作用域安装在感兴趣的蛋白质的末端。这些分子间相互作用可以通过选择 ID 来轻松调节,以控制材料相互作用和解吸能量,从而可以调节蛋白质释放动力学以适应所需的释放曲线。分子动力学研究提供了对控制释放机制的分子水平理解,并确定了凝胶原纤维上的最佳结合区域,这些区域促进了强ID-材料相互作用,这对于蛋白质的持续释放至关重要。该递送平台可以轻松装载货物,可植入剪切薄注射器,提供改进的蛋白质稳定性,能够实现多种体外释放速率,最重要的是,可以实现对体内蛋白质递送的长期控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号