当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Degradation of iridium oxides via oxygen evolution from the lattice: correlating atomic scale structure with reaction mechanisms†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2019-10-18 , DOI: 10.1039/c9ee01872g Olga Kasian 1, 2, 3, 4, 5 , Simon Geiger 4, 5, 6 , Tong Li 4, 5, 6, 7, 8 , Jan-Philipp Grote 4, 5, 6 , Kevin Schweinar 4, 5, 6 , Siyuan Zhang 4, 5, 6 , Christina Scheu 4, 5, 6 , Dierk Raabe 4, 5, 6 , Serhiy Cherevko 4, 9, 10, 11 , Baptiste Gault 4, 5, 6, 12, 13 , Karl J. J. Mayrhofer 4, 5, 6, 9, 10
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2019-10-18 , DOI: 10.1039/c9ee01872g Olga Kasian 1, 2, 3, 4, 5 , Simon Geiger 4, 5, 6 , Tong Li 4, 5, 6, 7, 8 , Jan-Philipp Grote 4, 5, 6 , Kevin Schweinar 4, 5, 6 , Siyuan Zhang 4, 5, 6 , Christina Scheu 4, 5, 6 , Dierk Raabe 4, 5, 6 , Serhiy Cherevko 4, 9, 10, 11 , Baptiste Gault 4, 5, 6, 12, 13 , Karl J. J. Mayrhofer 4, 5, 6, 9, 10
Affiliation
|
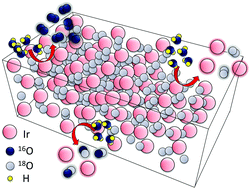
Understanding the fundamentals of iridium degradation during the oxygen evolution reaction is of importance for the development of efficient and durable water electrolysis systems. The degradation mechanism is complex and it is under intense discussion whether the oxygen molecule can be directly released from the oxide lattice. Here, we define the extent of lattice oxygen participation in the oxygen evolution and associated degradation of rutile and hydrous iridium oxide catalysts, and correlate this mechanism with the atomic-scale structures of the catalytic surfaces. We combine isotope labelling with atom probe tomography, online electrochemical and inductively coupled plasma mass spectrometry. Our data reveal that, unlike rutile IrO2, Ir hydrous oxide contains –IrIIIOOH species which directly contribute to the oxygen evolution from the lattice. This oxygen evolution mechanism results in faster degradation and dissolution of Ir. In addition, near surface bulk regions of hydrous oxide are involved in the oxygen catalysis and dissolution, while only the topmost atomic layers of rutile IrO2 participate in both reactions. Overall our data provide a contribution to the fundamental understanding of the exceptional stability of Ir-oxides towards the oxygen evolution reaction. The proposed approach to a quantitative assessment of the degree of lattice oxygen participation in the oxygen evolution reaction can be further applied to other oxide catalyst systems.
中文翻译:

铱氧化物的降解经由从晶格氧演化:原子尺度结构与反应机理相关†
了解氧释放反应过程中铱降解的基本原理对于开发高效耐用的水电解系统至关重要。降解机理很复杂,是否可以从氧化物晶格中直接释放出氧分子正处于激烈的讨论之中。在这里,我们定义了晶格中氧参与金红石型和水合氧化铱催化剂的氧气逸出和相关降解的程度,并将该机理与催化表面的原子尺度结构相关联。我们将同位素标记与原子探针层析成像,在线电化学和电感耦合等离子体质谱相结合。我们的数据表明,与金红石型IrO 2不同,Ir含水氧化物包含–Ir III直接导致氧从晶格逸出的OOH物种。这种氧气释放机理导致Ir更快地降解和溶解。另外,水合氧化物的近表面本体区域参与氧催化和溶解,而金红石型IrO 2的仅最顶层原子层参与两个反应。总的来说,我们的数据为对Ir氧化物对氧气释放反应的出色稳定性的基本理解做出了贡献。所提出的定量评估氧释放反应中晶格氧参与程度的方法可以进一步应用于其他氧化物催化剂体系。
更新日期:2019-12-04
中文翻译:

铱氧化物的降解经由从晶格氧演化:原子尺度结构与反应机理相关†
了解氧释放反应过程中铱降解的基本原理对于开发高效耐用的水电解系统至关重要。降解机理很复杂,是否可以从氧化物晶格中直接释放出氧分子正处于激烈的讨论之中。在这里,我们定义了晶格中氧参与金红石型和水合氧化铱催化剂的氧气逸出和相关降解的程度,并将该机理与催化表面的原子尺度结构相关联。我们将同位素标记与原子探针层析成像,在线电化学和电感耦合等离子体质谱相结合。我们的数据表明,与金红石型IrO 2不同,Ir含水氧化物包含–Ir III直接导致氧从晶格逸出的OOH物种。这种氧气释放机理导致Ir更快地降解和溶解。另外,水合氧化物的近表面本体区域参与氧催化和溶解,而金红石型IrO 2的仅最顶层原子层参与两个反应。总的来说,我们的数据为对Ir氧化物对氧气释放反应的出色稳定性的基本理解做出了贡献。所提出的定量评估氧释放反应中晶格氧参与程度的方法可以进一步应用于其他氧化物催化剂体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号