当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sphingolipid Modulation Activates Proteostasis Programs to Govern Human Hematopoietic Stem Cell Self-Renewal.
Cell Stem Cell ( IF 23.9 ) Pub Date : 2019-10-17 , DOI: 10.1016/j.stem.2019.09.008 Stephanie Z Xie 1 , Laura Garcia-Prat 1 , Veronique Voisin 2 , Robin Ferrari 1 , Olga I Gan 1 , Elvin Wagenblast 1 , Kerstin B Kaufmann 1 , Andy G X Zeng 3 , Shin-Ichiro Takayanagi 4 , Ishita Patel 1 , Esther K Lee 1 , Joseph Jargstorf 1 , Gareth Holmes 1 , Guy Romm 1 , Kristele Pan 1 , Michelle Shoong 1 , Aditi Vedi 5 , Chiara Luberto 6 , Mark D Minden 7 , Gary D Bader 8 , Elisa Laurenti 5 , John E Dick 3
Cell Stem Cell ( IF 23.9 ) Pub Date : 2019-10-17 , DOI: 10.1016/j.stem.2019.09.008 Stephanie Z Xie 1 , Laura Garcia-Prat 1 , Veronique Voisin 2 , Robin Ferrari 1 , Olga I Gan 1 , Elvin Wagenblast 1 , Kerstin B Kaufmann 1 , Andy G X Zeng 3 , Shin-Ichiro Takayanagi 4 , Ishita Patel 1 , Esther K Lee 1 , Joseph Jargstorf 1 , Gareth Holmes 1 , Guy Romm 1 , Kristele Pan 1 , Michelle Shoong 1 , Aditi Vedi 5 , Chiara Luberto 6 , Mark D Minden 7 , Gary D Bader 8 , Elisa Laurenti 5 , John E Dick 3
Affiliation
|
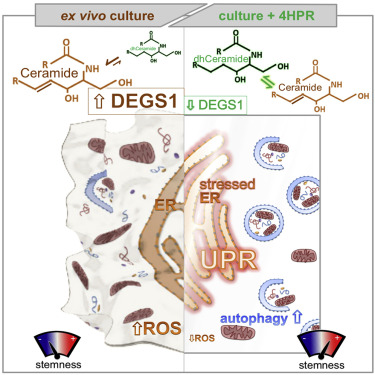
Cellular stress responses serve as crucial decision points balancing persistence or culling of hematopoietic stem cells (HSCs) for lifelong blood production. Although strong stressors cull HSCs, the linkage between stress programs and self-renewal properties that underlie human HSC maintenance remains unknown, particularly at quiescence exit when HSCs must also dynamically shift metabolic state. Here, we demonstrate distinct wiring of the sphingolipidome across the human hematopoietic hierarchy and find that genetic or pharmacologic modulation of the sphingolipid enzyme DEGS1 regulates lineage differentiation. Inhibition of DEGS1 in hematopoietic stem and progenitor cells during the transition from quiescence to cellular activation with N-(4-hydroxyphenyl) retinamide activates coordinated stress pathways that coalesce on endoplasmic reticulum stress and autophagy programs to maintain immunophenotypic and functional HSCs. Thus, our work identifies a linkage between sphingolipid metabolism, proteostatic quality control systems, and HSC self-renewal and provides therapeutic targets for improving HSC-based cellular therapeutics.
中文翻译:

鞘脂调节激活蛋白质稳态程序来控制人类造血干细胞的自我更新。
细胞应激反应是平衡造血干细胞 (HSC) 的持久性或终生造血的剔除的关键决策点。尽管强压力源会淘汰 HSC,但人类 HSC 维持的压力程序与自我更新特性之间的联系仍然未知,特别是在静息退出时,HSC 还必须动态改变代谢状态。在这里,我们证明了鞘脂组在人类造血系统中的独特接线,并发现鞘脂酶 DEGS1 的遗传或药理学调节可调节谱系分化。在造血干细胞和祖细胞从静止到细胞激活的转变过程中,用 N-(4-羟苯基) 视黄酰胺抑制 DEGS1 会激活协调的应激途径,该途径联合内质网应激和自噬程序,以维持 HSC 的免疫表型和功能。因此,我们的工作确定了鞘脂代谢、蛋白质量控制系统和 HSC 自我更新之间的联系,并为改进基于 HSC 的细胞疗法提供了治疗靶点。
更新日期:2019-11-09
中文翻译:

鞘脂调节激活蛋白质稳态程序来控制人类造血干细胞的自我更新。
细胞应激反应是平衡造血干细胞 (HSC) 的持久性或终生造血的剔除的关键决策点。尽管强压力源会淘汰 HSC,但人类 HSC 维持的压力程序与自我更新特性之间的联系仍然未知,特别是在静息退出时,HSC 还必须动态改变代谢状态。在这里,我们证明了鞘脂组在人类造血系统中的独特接线,并发现鞘脂酶 DEGS1 的遗传或药理学调节可调节谱系分化。在造血干细胞和祖细胞从静止到细胞激活的转变过程中,用 N-(4-羟苯基) 视黄酰胺抑制 DEGS1 会激活协调的应激途径,该途径联合内质网应激和自噬程序,以维持 HSC 的免疫表型和功能。因此,我们的工作确定了鞘脂代谢、蛋白质量控制系统和 HSC 自我更新之间的联系,并为改进基于 HSC 的细胞疗法提供了治疗靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号