当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming synthetic challenges in targeting coenzyme A biosynthesis with the antimicrobial natural product CJ-15,801.
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019-10-17 , DOI: 10.1039/c9md00312f Riyad Domingo 1 , Renier van der Westhuyzen 1 , Anton R Hamann 1 , Konrad J Mostert 1 , Leanne Barnard 1 , Tanya Paquet 1 , Erick T Tjhin 2 , Kevin J Saliba 2, 3 , Willem A L van Otterlo 4 , Erick Strauss 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019-10-17 , DOI: 10.1039/c9md00312f Riyad Domingo 1 , Renier van der Westhuyzen 1 , Anton R Hamann 1 , Konrad J Mostert 1 , Leanne Barnard 1 , Tanya Paquet 1 , Erick T Tjhin 2 , Kevin J Saliba 2, 3 , Willem A L van Otterlo 4 , Erick Strauss 1
Affiliation
|
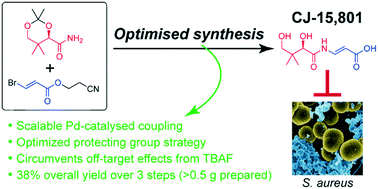
The biosynthesis of the essential metabolic cofactor coenzyme A (CoA) has been receiving increasing attention as a new target that shows potential to counter the rising resistance to established antimicrobials. In particular, phosphopantothenoylcysteine synthetase (PPCS)-the second CoA biosynthesis enzyme that is found as part of the bifunctional CoaBC protein in bacteria, but is monofunctional in eukaryotes-has been validated as a target through extensive genetic knockdown studies in Mycobacterium tuberculosis. Moreover, it has been identified as the molecular target of the fungal natural product CJ-15,801 that shows selective activity against Staphylococcus aureus and the malaria parasite Plasmodium falciparum. As such, CJ-15,801 and 4'-phospho-CJ-15,801 (its metabolically active form) are excellent tool compounds for use in the development of new antimicrobial PPCS inhibitors. Unfortunately, further study and analysis of CJ-15,801 is currently being hampered by several unique challenges posed by its synthesis. In this study we describe how these challenges were overcome by using a robust palladium-catalyzed coupling to form the key N-acyl vinylogous carbamate moiety with retention of stereochemistry, and by extensive investigation of protecting groups suited to the labile functional group combinations contained in this molecule. We also demonstrate that using TBAF for deprotection causes undesired off-target effects related to the presence of residual tertiary ammonium salts. Finally, we provide a new method for the chemoenzymatic preparation of 4'-phospho-CJ-15,801 on multi-milligram scale, after showing that chemical synthesis of the molecule is not practical. Taken together, the results of this study advances our pursuit to discover new antimicrobials that specifically target CoA biosynthesis and/or utilization.
中文翻译:

用抗菌天然产物 CJ-15,801 克服靶向辅酶 A 生物合成的合成挑战。
必需代谢辅因子辅酶 A (CoA) 的生物合成作为一个新靶点受到越来越多的关注,该靶点显示出对抗对已确立的抗菌药物耐药性上升的潜力。特别是,磷酸泛酰半胱氨酸合成酶 (PPCS)——第二种 CoA 生物合成酶,被发现是细菌中双功能 CoaBC 蛋白的一部分,但在真核生物中是单功能的——已通过对结核分枝杆菌的广泛基因敲除研究被证实为靶标。此外,它已被确定为真菌天然产物 CJ-15,801 的分子靶标,对金黄色葡萄球菌和疟原虫恶性疟原虫具有选择性活性。因此,CJ-15,801 和 4'-磷酸-CJ-15,801(其代谢活性形式)是用于开发新型抗菌 PPCS 抑制剂的优秀工具化合物。不幸的是,CJ-15,801 的进一步研究和分析目前受到其合成所带来的几个独特挑战的阻碍。在这项研究中,我们描述了如何通过使用稳健的钯催化偶联形成具有立体化学保留的关键 N-酰基乙烯基氨基甲酸酯部分来克服这些挑战,并通过广泛研究适合于其中包含的不稳定官能团组合的保护基团分子。我们还证明,使用 TBAF 进行脱保护会导致与残留叔铵盐的存在相关的不希望的脱靶效应。最后,我们提供了一种化学酶法制备4'-磷酸-CJ-15的新方法,801 在数毫克规模上,表明该分子的化学合成是不切实际的。总之,这项研究的结果推动了我们发现专门针对 CoA 生物合成和/或利用的新抗菌剂的追求。
更新日期:2019-12-13
中文翻译:

用抗菌天然产物 CJ-15,801 克服靶向辅酶 A 生物合成的合成挑战。
必需代谢辅因子辅酶 A (CoA) 的生物合成作为一个新靶点受到越来越多的关注,该靶点显示出对抗对已确立的抗菌药物耐药性上升的潜力。特别是,磷酸泛酰半胱氨酸合成酶 (PPCS)——第二种 CoA 生物合成酶,被发现是细菌中双功能 CoaBC 蛋白的一部分,但在真核生物中是单功能的——已通过对结核分枝杆菌的广泛基因敲除研究被证实为靶标。此外,它已被确定为真菌天然产物 CJ-15,801 的分子靶标,对金黄色葡萄球菌和疟原虫恶性疟原虫具有选择性活性。因此,CJ-15,801 和 4'-磷酸-CJ-15,801(其代谢活性形式)是用于开发新型抗菌 PPCS 抑制剂的优秀工具化合物。不幸的是,CJ-15,801 的进一步研究和分析目前受到其合成所带来的几个独特挑战的阻碍。在这项研究中,我们描述了如何通过使用稳健的钯催化偶联形成具有立体化学保留的关键 N-酰基乙烯基氨基甲酸酯部分来克服这些挑战,并通过广泛研究适合于其中包含的不稳定官能团组合的保护基团分子。我们还证明,使用 TBAF 进行脱保护会导致与残留叔铵盐的存在相关的不希望的脱靶效应。最后,我们提供了一种化学酶法制备4'-磷酸-CJ-15的新方法,801 在数毫克规模上,表明该分子的化学合成是不切实际的。总之,这项研究的结果推动了我们发现专门针对 CoA 生物合成和/或利用的新抗菌剂的追求。











































 京公网安备 11010802027423号
京公网安备 11010802027423号