当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The detection of the precursors of the photorearranged products of 3-hydroxyflavones in selected solvents from UV-visible spectra in situ.
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2019-10-29 , DOI: 10.1039/c9pp00316a Jyoti Tomar 1 , Kulvir Kaur 1 , Manisha Bansal 1
Photochemical & Photobiological Sciences ( IF 2.7 ) Pub Date : 2019-10-29 , DOI: 10.1039/c9pp00316a Jyoti Tomar 1 , Kulvir Kaur 1 , Manisha Bansal 1
Affiliation
|
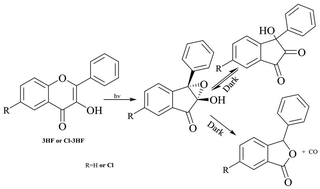
Mechanistic studies relating to the photochemistry of 3-hydroxy-2-phenyl-4H-chromen-4-one (3HF) and 6-chloro-3-hydroxy-2-phenyl-4H-chromen-4-one (Cl-3HF) have been reinvestigated in selected solvents. The UV-visible spectra of the photoproduct(s) of 3HF and Cl-3HF have been computed in situ via subtracting the spectra of unreacted substrates, with acetonitrile (ACN) and methanol (MeOH) as solvents. These spectra turn out to be different from the spectra of the corresponding isolated photoproducts: 3-hydroxy-3-phenyl-indan-1,2-dione and 6-chloro-3-hydroxy-3-phenyl-indan-1,2-dione (referred to as dione ). Analyses of the photoproduct(s) via GC-MS show the formation of a single detectable product, i.e., the corresponding dione. On the basis of some experimental observations, it is proposed that the primary photoproduct in situ is 2,3-epoxy-2-hydroxy-1-indanone (referred to as epoxide) instead of dione as reported in previous years. Earlier, epoxide has been proposed to be the intermediate in the mechanism for the formation of dione. This is the first report where the formation of epoxide has been directly detected in the selected solvents. On the other hand, both dione and epoxide (2 : 1) are shown to be formed with MeOH as solvent. The second important finding is that epoxide and dione interconvert in the dark, depending upon the environment. With ACN as solvent, pure dione in the dark is kinetically and partially converted to epoxide. With MeOH as solvent, epoxide is instantly and partially converted to dione until both are in equilibrium. However, a solution of dione in MeOH remains stable in the dark. The photoformation of epoxide is quantitative with ACN as solvent and it is sufficiently stable. It has been further observed that epoxide solutions of 3HF and Cl-3HF in ACN are quantitatively converted into 3-phenylisobenzofuran-1(3H)-one and 6-chloro-3-phenylisobenzofuran-1(3H)-one, i.e., the corresponding phthalides, through the loss of CO when kept in the dark for some days. A mechanism has been proposed where epoxide has been shown to give dione and/or phthalide via selective C-O or C-C bond cleavage in the oxiranyl ring, respectively. The selection of this cleavage depends mainly on the solvent system and the substituents in the parent flavones.
中文翻译:

从紫外可见光谱中检测所选溶剂中3-羟基黄酮的光重排产物的前体。
与3-羟基-2-苯基-4H-铬-4-酮(3HF)和6-氯-3-羟基-2-苯基-4H-铬-4-酮(Cl-3HF)的光化学有关的机理研究已在选定的溶剂中进行了重新研究。通过用乙腈(ACN)和甲醇(MeOH)作为溶剂减去未反应的底物的光谱,就地计算出了3HF和Cl-3HF的光产物的UV-可见光谱。这些光谱与相应的分离的光产物的光谱不同:3-羟基-3-苯基-茚满-1,2-二酮和6-氯-3-羟基-3-苯基-茚满-1,2- dione(简称dione)。通过GC-MS对光产物的分析表明形成了单个可检测产物,即相应的二酮。根据一些实验观察结果,建议原位光产物为2,3-环氧-2-羟基-1-茚满酮(称为环氧化物)代替了前几年报道的二酮。早先,有人提出环氧化物是二酮形成机理的中间产物。这是首次在所选溶剂中直接检测到环氧化物形成的报告。另一方面,显示出用MeOH作为溶剂形成二酮和环氧化物(2∶1)。第二个重要发现是环氧化物和二酮在黑暗中互变,具体取决于环境。使用ACN作为溶剂,黑暗中的纯二酮在动力学上部分转化为环氧化物。以MeOH为溶剂,环氧化物立即部分转化为二酮,直到两者均处于平衡状态。然而,二酮在MeOH中的溶液在黑暗中保持稳定。以乙腈为溶剂,环氧化物的光形成是定量的,并且足够稳定。进一步观察到,在ACN中3HF和Cl-3HF的环氧溶液被定量地转化为3-苯基异苯并呋喃-1(3H)-one和6-氯-3-苯基异苯并呋喃-1(3H)-one,即相应的邻苯二甲酸盐,在黑暗中放置几天会失去一氧化碳。已经提出了一种机理,其中已经表明环氧化物分别通过环氧乙烷基环中的选择性CO或CC键裂解产生二酮和/或邻苯二甲酸酯。该裂解的选择主要取决于溶剂体系和母体黄酮中的取代基。进一步观察到,在ACN中3HF和Cl-3HF的环氧溶液被定量地转化为3-苯基异苯并呋喃-1(3H)-one和6-氯-3-苯基异苯并呋喃-1(3H)-one,即相应的邻苯二甲酸盐,在黑暗中放置几天会失去一氧化碳。已经提出了一种机理,其中已经表明环氧化物分别通过环氧乙烷基环中的选择性CO或CC键裂解产生二酮和/或邻苯二甲酸酯。该裂解的选择主要取决于溶剂体系和母体黄酮中的取代基。进一步观察到,在ACN中3HF和Cl-3HF的环氧溶液被定量地转化为3-苯基异苯并呋喃-1(3H)-one和6-氯-3-苯基异苯并呋喃-1(3H)-one,即相应的邻苯二甲酸盐,在黑暗中放置几天会失去一氧化碳。已经提出了一种机理,其中已经表明环氧化物分别通过环氧乙烷基环中的选择性CO或CC键裂解产生二酮和/或邻苯二甲酸酯。该裂解的选择主要取决于溶剂体系和母体黄酮中的取代基。
更新日期:2020-01-01
中文翻译:

从紫外可见光谱中检测所选溶剂中3-羟基黄酮的光重排产物的前体。
与3-羟基-2-苯基-4H-铬-4-酮(3HF)和6-氯-3-羟基-2-苯基-4H-铬-4-酮(Cl-3HF)的光化学有关的机理研究已在选定的溶剂中进行了重新研究。通过用乙腈(ACN)和甲醇(MeOH)作为溶剂减去未反应的底物的光谱,就地计算出了3HF和Cl-3HF的光产物的UV-可见光谱。这些光谱与相应的分离的光产物的光谱不同:3-羟基-3-苯基-茚满-1,2-二酮和6-氯-3-羟基-3-苯基-茚满-1,2- dione(简称dione)。通过GC-MS对光产物的分析表明形成了单个可检测产物,即相应的二酮。根据一些实验观察结果,建议原位光产物为2,3-环氧-2-羟基-1-茚满酮(称为环氧化物)代替了前几年报道的二酮。早先,有人提出环氧化物是二酮形成机理的中间产物。这是首次在所选溶剂中直接检测到环氧化物形成的报告。另一方面,显示出用MeOH作为溶剂形成二酮和环氧化物(2∶1)。第二个重要发现是环氧化物和二酮在黑暗中互变,具体取决于环境。使用ACN作为溶剂,黑暗中的纯二酮在动力学上部分转化为环氧化物。以MeOH为溶剂,环氧化物立即部分转化为二酮,直到两者均处于平衡状态。然而,二酮在MeOH中的溶液在黑暗中保持稳定。以乙腈为溶剂,环氧化物的光形成是定量的,并且足够稳定。进一步观察到,在ACN中3HF和Cl-3HF的环氧溶液被定量地转化为3-苯基异苯并呋喃-1(3H)-one和6-氯-3-苯基异苯并呋喃-1(3H)-one,即相应的邻苯二甲酸盐,在黑暗中放置几天会失去一氧化碳。已经提出了一种机理,其中已经表明环氧化物分别通过环氧乙烷基环中的选择性CO或CC键裂解产生二酮和/或邻苯二甲酸酯。该裂解的选择主要取决于溶剂体系和母体黄酮中的取代基。进一步观察到,在ACN中3HF和Cl-3HF的环氧溶液被定量地转化为3-苯基异苯并呋喃-1(3H)-one和6-氯-3-苯基异苯并呋喃-1(3H)-one,即相应的邻苯二甲酸盐,在黑暗中放置几天会失去一氧化碳。已经提出了一种机理,其中已经表明环氧化物分别通过环氧乙烷基环中的选择性CO或CC键裂解产生二酮和/或邻苯二甲酸酯。该裂解的选择主要取决于溶剂体系和母体黄酮中的取代基。进一步观察到,在ACN中3HF和Cl-3HF的环氧溶液被定量地转化为3-苯基异苯并呋喃-1(3H)-one和6-氯-3-苯基异苯并呋喃-1(3H)-one,即相应的邻苯二甲酸盐,在黑暗中放置几天会失去一氧化碳。已经提出了一种机理,其中已经表明环氧化物分别通过环氧乙烷基环中的选择性CO或CC键裂解产生二酮和/或邻苯二甲酸酯。该裂解的选择主要取决于溶剂体系和母体黄酮中的取代基。









































 京公网安备 11010802027423号
京公网安备 11010802027423号