当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ab Initio Calculation of Equilibrium Isotopic Fractionations of Potassium and Rubidium in Minerals and Water
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-11-04 , DOI: 10.1021/acsearthspacechem.9b00180 Hao Zeng , Viktor F. Rozsa , Nicole Xike Nie , Zhe Zhang , Tuan Anh Pham 1 , Giulia Galli 2, 3 , Nicolas Dauphas
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-11-04 , DOI: 10.1021/acsearthspacechem.9b00180 Hao Zeng , Viktor F. Rozsa , Nicole Xike Nie , Zhe Zhang , Tuan Anh Pham 1 , Giulia Galli 2, 3 , Nicolas Dauphas
Affiliation
|
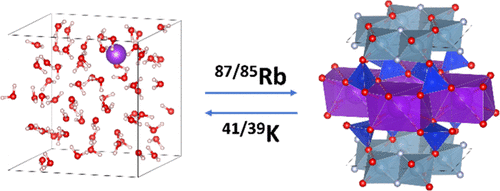
We used first-principle approaches to calculate the equilibrium isotopic fractionation factors of potassium (K) and rubidium (Rb) in a variety of minerals of geological relevance (orthoclase, albite, muscovite, illite, sylvite, and phlogopite). We also used molecular dynamics simulations to calculate the equilibrium isotopic fractionation factors of K in water. Our results indicate that K and Rb form bonds of similar strengths and that the ratio between the equilibrium fractionations of K and Rb is approximately 3–4. Under low-temperature conditions relevant to weathering of continents or alteration of seafloor basalts (∼25 °C), the K isotopic fractionation between solvated K+ and illite (a proxy for K-bearing clays) is +0.24‰, exceeding the current analytical precision, so equilibrium isotopic fractionation can induce measurable isotopic fractionations for this system at low temperature. These findings, however, cannot easily explain why the δ41K value of seawater is shifted by +0.6‰ relative to igneous rocks. Our results indicate that part of the observed fractionation is most likely due to kinetic effects. The narrow range of mean force constants for K and Rb in silicate minerals suggests that phase equilibrium is unlikely to create large K and Rb isotopic fractionations at magmatic temperatures (at least in silicate systems). Kinetic effects associated with diffusion can, however, produce large K and Rb isotopic fractionations in igneous rocks.
中文翻译:

矿物质和水中钾和Rub平衡同位素分数的从头算
我们使用第一性原理方法来计算各种地质相关矿物(正长石,钠长石,白云母,伊利石,钾盐和金云母)中钾(K)和rub(Rb)的平衡同位素分馏因子。我们还使用分子动力学模拟来计算水中K的平衡同位素分馏因子。我们的结果表明,K和Rb形成强度相似的键,并且K和Rb的平衡分数之比约为3-4。在与大陆风化或海底玄武岩变化有关的低温条件下(约25°C),溶剂化的K +之间的K同位素分馏伊利石(含K粘土的代用品)为+ 0.24‰,超过了目前的分析精度,因此在低温下该系统的平衡同位素分馏可以诱导出可测量的同位素分馏。这些发现,然而,不能容易地解释为什么δ 41海水的K值是由+ 0.6‰相对于火成岩偏移。我们的结果表明,观察到的部分馏分最有可能是由于动力学作用。硅酸盐矿物中K和Rb的平均力常数范围狭窄,这表明相平衡不太可能在岩浆温度下(至少在硅酸盐体系中)产生较大的K和Rb同位素分馏。然而,与扩散有关的动力学效应会在火成岩中产生大量的K和Rb同位素分馏。
更新日期:2019-11-04
中文翻译:

矿物质和水中钾和Rub平衡同位素分数的从头算
我们使用第一性原理方法来计算各种地质相关矿物(正长石,钠长石,白云母,伊利石,钾盐和金云母)中钾(K)和rub(Rb)的平衡同位素分馏因子。我们还使用分子动力学模拟来计算水中K的平衡同位素分馏因子。我们的结果表明,K和Rb形成强度相似的键,并且K和Rb的平衡分数之比约为3-4。在与大陆风化或海底玄武岩变化有关的低温条件下(约25°C),溶剂化的K +之间的K同位素分馏伊利石(含K粘土的代用品)为+ 0.24‰,超过了目前的分析精度,因此在低温下该系统的平衡同位素分馏可以诱导出可测量的同位素分馏。这些发现,然而,不能容易地解释为什么δ 41海水的K值是由+ 0.6‰相对于火成岩偏移。我们的结果表明,观察到的部分馏分最有可能是由于动力学作用。硅酸盐矿物中K和Rb的平均力常数范围狭窄,这表明相平衡不太可能在岩浆温度下(至少在硅酸盐体系中)产生较大的K和Rb同位素分馏。然而,与扩散有关的动力学效应会在火成岩中产生大量的K和Rb同位素分馏。











































 京公网安备 11010802027423号
京公网安备 11010802027423号