The Lancet Respiratory Medicine ( IF 38.7 ) Pub Date : 2019-10-15 , DOI: 10.1016/s2213-2600(19)30252-8 Yunpeng Yang 1 , Jianya Zhou 2 , Jianying Zhou 2 , Jifeng Feng 3 , Wu Zhuang 4 , Jianhua Chen 5 , Jun Zhao 6 , Wei Zhong 7 , Yanqiu Zhao 8 , Yiping Zhang 9 , Yong Song 10 , Yi Hu 11 , Zhuang Yu 12 , Youling Gong 13 , Yuan Chen 14 , Feng Ye 15 , Shucai Zhang 16 , Lejie Cao 17 , Yun Fan 9 , Gang Wu 18 , Yubiao Guo 19 , Chengzhi Zhou 20 , Kewei Ma 21 , Jian Fang 6 , Weineng Feng 22 , Yunpeng Liu 23 , Zhendong Zheng 24 , Gaofeng Li 25 , Ning Wu 26 , Wei Song 27 , Xiaoqing Liu 28 , Shijun Zhao 26 , Lieming Ding 29 , Li Mao 29 , Giovanni Selvaggi 30 , Xiaobin Yuan 29 , Yuanqing Fu 29 , Tao Wang 31 , Shanshan Xiao 31 , Li Zhang 1
|
Background
Ensartinib is a potent new-generation ALK inhibitor with high activity against a broad range of known crizotinib-resistant ALK mutations and CNS metastases. We aimed to assess the efficacy and safety of ensartinib in ALK-positive patients with non-small-cell lung cancer (NSCLC), in whom crizotinib therapy was unsuccessful. The associations between ensartinib efficacy and crizotinib-resistant mutations were also explored.
Methods
We did a single-arm, open-label, phase 2 study at 27 centres in China. Patients were aged 18 years or older, had stage IIIb or stage IV ALK-positive NSCLC that had progressed while they were on crizotinib therapy, an Eastern Cooperative Oncology Group performance status of 2 or less, had measurable disease, and had received fewer than three previous treatments. Patients with CNS metastases were included if these metastases were asymptomatic and did not require steroid therapy. All patients received 225 mg ensartinib orally once daily on a continuous dosing schedule. The primary outcome was the proportion of patients with an objective response according to the Response Evaluation Criteria in Solid Tumors (version 1.1), as assessed by an independent review committee in all patients who received at least one dose of ensartinib with no major violations of the inclusion criteria (ie, the full analysis set). Safety was assessed in all enrolled patients who received at least one dose of ensartinib. This trial was registered with ClinicalTrials.gov, NCT03215693.
Findings
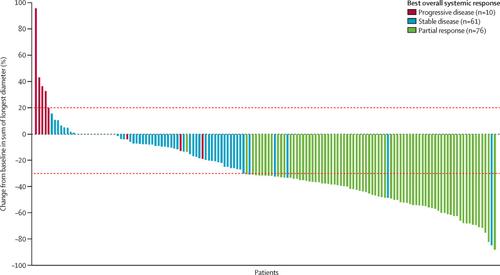
Between Sept 28, 2017, and April 11, 2018, 160 patients were enrolled and had at least one dose of ensartinib (safety analysis set). Four patients had inclusion violations and were excluded from the efficacy analysis, which thus included 156 patients (full analysis set). 97 (62%) patients in the full analysis set had brain metastases. 76 (52% [95% CI 43–60]) of 147 patients in the full analysis set, with responses that could be assessed by the independent review committee, had an objective response. 28 (70% [53–83]) of 40 patients with measurable brain metastases as assessed by the independent review committee had an intracranial objective response. 145 (91%) of 160 patients had at least one treatment-related adverse event, which were mostly grade 1 or 2. The most common treatment-related adverse events were rash (89 [56%]), increased alanine aminotransferase concentrations (74 [46%]), and increased aspartate aminotransferase concentrations (65 [41%]).
Interpretation
Ensartinib has activity and is well tolerated in patients with crizotinib-refractory, ALK-positive NSCLC, including those with brain metastases. The role of ensartinib in patients in whom other second-generation ALK inhibitors have been unsuccessful warrants further studies.
Funding
Betta Pharmaceuticals.











































 京公网安备 11010802027423号
京公网安备 11010802027423号