European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2019-10-14 , DOI: 10.1016/j.ejpb.2019.10.002 Mahbubur Rahman , Faustin Arevalo , Alexander Coelho , Ecevit Bilgili
|
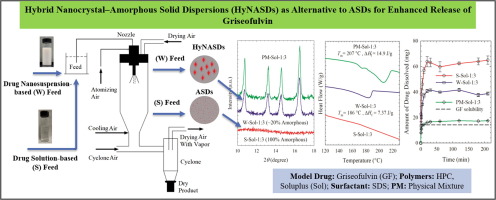
A major shortcoming of drug nanocomposites as compared with amorphous solid dispersions (ASDs) is their limited supersaturation capability in dissolution media. Here, we prepared drug hybrid nanocrystal–amorphous solid dispersions (HyNASDs) and compare their performance to ASDs. A wet-milled griseofulvin (GF, BCS II drug) nanosuspension and a GF solution, both containing the same dissolved polymer–surfactant (SDS: sodium dodecyl sulfate) with 1:1 and 1:3 GF:polymer mass ratios, were spray-dried. Hydroxypropyl cellulose (HPC) and Soluplus (Sol) were used as matrix-forming polymers. XRPD, DSC, and Raman spectroscopy reveal that ASDs were formed upon spray-drying the solution-based feed, whereas nanocomposites and nanocomposites with >10% amorphous content, HyNASDs, were formed with the nanosuspension-based feed. Sol provided higher GF relative supersaturation, up to 180% and 360% for HyNASDs and ASDs, respectively, in the dissolution tests than HPC (up to 50% for both) owing to Sol’s stronger intermolecular interactions and miscibility with GF and its recrystallization inhibition. Besides the higher kinetic solubility of GF in Sol, presence of GF nanoparticles vs. micron-sized particles in the nanocomposites enabled fast supersaturation. This study demonstrates successful preparation of fast supersaturating (190% within 20 min) HyNASDs, which renders nanoparticle formulations competitive to ASDs in bioavailability enhancement of poorly soluble drugs.
中文翻译:

杂化纳米晶体-无定形固体分散体(HyNASD)替代ASD以增强BCS II类药物的释放
与无定形固体分散体(ASD)相比,药物纳米复合材料的主要缺点是其在溶出介质中的过饱和能力有限。在这里,我们制备了药物杂化纳米晶体-无定形固体分散体(HyNASD),并将其性能与ASD进行了比较。将湿磨的灰黄霉素(GF,BCS II药物)纳米悬浮液和GF溶液喷雾-均含有相同的溶解的聚合物-表面活性剂(SDS:十二烷基硫酸钠),GF:聚合物的质量比为1:1和1:3。干的。羟丙基纤维素(HPC)和Soluplus(Sol)被用作形成基质的聚合物。XRPD,DSC和拉曼光谱表明,ASD是在喷雾干燥基于溶液的饲料时形成的,而纳米复合材料和具有10%以上无定形含量的纳米复合材料HyNASDs是由基于纳米悬浮液的饲料形成的。Sol的溶出度测试中,HyNASDs和ASD的GF相对过饱和度更高,分别比HPC高达180%和360%(两者均高达50%),这是因为Sol的分子间相互作用更强,并且与GF混溶并具有重结晶抑制作用。除了GF在溶胶中的较高的动力学溶解度之外,纳米复合材料中GF纳米粒子与微米级粒子的存在还实现了快速过饱和。这项研究表明成功制备了快速过饱和(20分钟内190%)的HyNASD,这使纳米颗粒制剂在难溶性药物的生物利用度增强方面与ASD竞争。由于Sol的分子间相互作用以及与GF的混溶性及其对重结晶的抑制作用,因此在溶出度测试中比HPC(两者均高达50%)高。除了GF在溶胶中的较高的动力学溶解度之外,纳米复合材料中GF纳米粒子与微米级粒子的存在还实现了快速过饱和。这项研究表明成功制备了快速过饱和(20分钟内190%)的HyNASD,这使纳米颗粒制剂在难溶性药物的生物利用度增强方面与ASD竞争。由于Sol的分子间相互作用以及与GF的混溶性及其对重结晶的抑制作用,因此在溶出度测试中比HPC(两者均高达50%)高。除了GF在溶胶中的较高的动力学溶解度之外,纳米复合材料中GF纳米粒子与微米级粒子的存在还实现了快速过饱和。这项研究表明成功制备了快速过饱和(20分钟内190%)的HyNASD,这使纳米颗粒制剂在难溶性药物的生物利用度增强方面与ASD竞争。









































 京公网安备 11010802027423号
京公网安备 11010802027423号