Nature Catalysis ( IF 42.8 ) Pub Date : 2019-10-14 , DOI: 10.1038/s41929-019-0351-2 Rory Little , Fernanda C. R. Paiva , Robert Jenkins , Hui Hong , Yuhui Sun , Yuliya Demydchuk , Markiyan Samborskyy , Manuela Tosin , Finian J. Leeper , Marcio V. B. Dias , Peter F. Leadlay
|
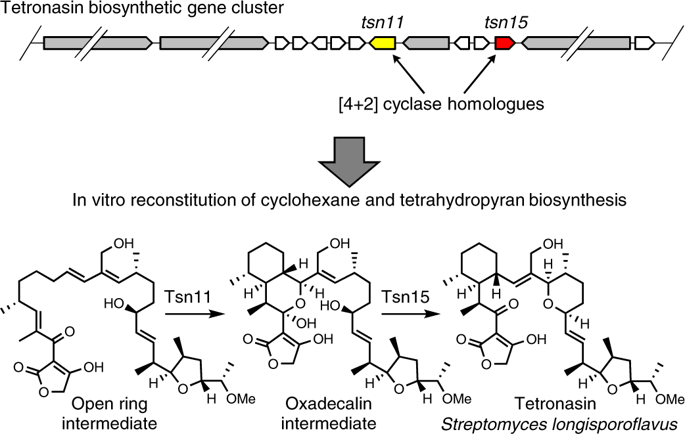
Enzymes that catalyse remarkable Diels–Alder-like [4+2] cyclizations have been previously implicated in the biosynthesis of spirotetronate and spirotetramate antibiotics. Biosynthesis of the polyether antibiotic tetronasin is not expected to require such steps, yet the tetronasin gene cluster encodes enzymes Tsn11 and Tsn15, which are homologous to authentic [4+2] cyclases. Here, we show that deletion of Tsn11 led to accumulation of a late-stage intermediate, in which the two central rings of tetronasin and four of its twelve asymmetric centres remain unformed. In vitro reconstitution showed that Tsn11 catalyses an apparent inverse-electron-demand hetero-Diels–Alder-like [4+2] cyclization of this species to form an unexpected oxadecalin compound that is then rearranged by Tsn15 to form tetronasin. To gain structural and mechanistic insight into the activity of Tsn15, the crystal structure of a Tsn15-substrate complex has been solved at 1.7 Å resolution.
中文翻译:

聚醚类抗生素生物合成中意外的酶催化的[4 + 2]环加成和重排
以前,催化拟狄尔斯-阿尔德[4 + 2]环化反应的酶与螺菌酯和螺四甲酸酯类抗生素的生物合成有关。聚醚抗生素替特罗辛的生物合成预计不需要这些步骤,但替特罗辛基因簇编码的酶Tsn11和Tsn15与真正的[4 + 2]环化酶同源。在这里,我们显示Tsn11的缺失导致后期中间体的积累,其中四氢钙蛋白的两个中心环及其十二个不对称中心中的四个仍未形成。体外重建显示,Tsn11催化该物种的明显逆电子需求的异Diels–Alder样[4 + 2]环化反应,形成意想不到的奥卡德林化合物,然后被Tsn15重排形成四氢松黄素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号