当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of a New Type I Baeyer-Villiger Monooxygenase from Amycolatopsis thermoflava Revealed High Thermodynamic but Limited Kinetic Stability.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/cbic.201900501 Hamid R Mansouri 1 , Marko D Mihovilovic 1 , Florian Rudroff 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/cbic.201900501 Hamid R Mansouri 1 , Marko D Mihovilovic 1 , Florian Rudroff 1
Affiliation
|
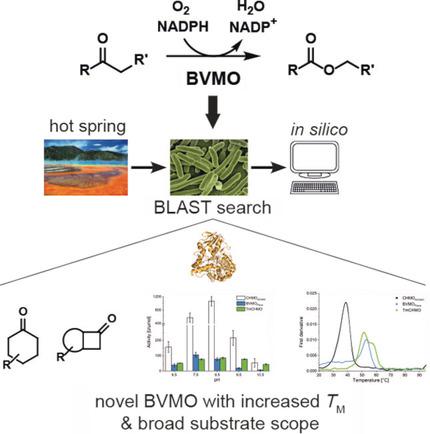
Baeyer-Villiger monooxygenases (BVMOs) are remarkable biocatalysts, but, due to their low stability, their application in industry is hampered. Thus, there is a high demand to expand on the diversity and increase the stability of this class of enzyme. Starting from a known thermostable BVMO sequence from Thermocrispum municipale (TmCHMO), a novel BVMO from Amycolaptosis thermoflava (BVMOFlava ), which was successfully expressed in Escherichia coli BL21(DE3), was identified. The activity and stability of the purified enzyme was investigated and the substrate profile for structurally different cyclohexanones and cyclobutanones was assigned. The enzyme showed a lower activity than that of cyclohexanone monooxygenase (CHMOAcineto ) from Acinetobacter sp., as the prototype BVMO, but indicated higher kinetic stability by showing a twofold longer half-life at 30 °C. The thermodynamic stability, as represented by the melting temperature, resulted in a Tm value of 53.1 °C for BVMOFlava , which was comparable to the Tm of TmCHMO (ΔTm =1 °C) and significantly higher than the Tm value for CHMOAcineto ((ΔTm =14.6 °C)). A strong deviation between the thermodynamic and kinetic stabilities of BVMOFlava was observed; this might have a major impact on future enzyme discovery for BVMOs and their synthetic applications.
中文翻译:

对来自热黄无枝酸菌的新型 I 型 Baeyer-Villiger 单加氧酶的研究揭示了热力学高但动力学稳定性有限。
拜尔-维利格单加氧酶(BVMO)是卓越的生物催化剂,但由于其稳定性低,其在工业中的应用受到阻碍。因此,迫切需要扩大此类酶的多样性并提高其稳定性。从来自 Thermocrispum 市政 (TmCHMO) 的已知热稳定性 BVMO 序列出发,鉴定了来自热黄支链霉 (BVMOFlava) 的新型 BVMO,该 BVMO 已在大肠杆菌 BL21(DE3) 中成功表达。研究了纯化酶的活性和稳定性,并指定了结构不同的环己酮和环丁酮的底物谱。该酶的活性低于来自不动杆菌属的环己酮单加氧酶(CHMOAcineto)(作为原型 BVMO),但在 30 °C 下半衰期延长了一倍,从而表现出更高的动力学稳定性。以熔化温度表示的热力学稳定性导致 BVMOFLava 的 Tm 值为 53.1 °C,与 TmCHMO (ΔTm =1 °C) 的 Tm 相当,并且显着高于 CHMOAcineto 的 Tm 值 ((ΔTm =14.6°C))。观察到 BVMOFlava 的热力学和动力学稳定性之间存在很大偏差;这可能会对未来 BVMO 酶的发现及其合成应用产生重大影响。
更新日期:2020-01-09
中文翻译:

对来自热黄无枝酸菌的新型 I 型 Baeyer-Villiger 单加氧酶的研究揭示了热力学高但动力学稳定性有限。
拜尔-维利格单加氧酶(BVMO)是卓越的生物催化剂,但由于其稳定性低,其在工业中的应用受到阻碍。因此,迫切需要扩大此类酶的多样性并提高其稳定性。从来自 Thermocrispum 市政 (TmCHMO) 的已知热稳定性 BVMO 序列出发,鉴定了来自热黄支链霉 (BVMOFlava) 的新型 BVMO,该 BVMO 已在大肠杆菌 BL21(DE3) 中成功表达。研究了纯化酶的活性和稳定性,并指定了结构不同的环己酮和环丁酮的底物谱。该酶的活性低于来自不动杆菌属的环己酮单加氧酶(CHMOAcineto)(作为原型 BVMO),但在 30 °C 下半衰期延长了一倍,从而表现出更高的动力学稳定性。以熔化温度表示的热力学稳定性导致 BVMOFLava 的 Tm 值为 53.1 °C,与 TmCHMO (ΔTm =1 °C) 的 Tm 相当,并且显着高于 CHMOAcineto 的 Tm 值 ((ΔTm =14.6°C))。观察到 BVMOFlava 的热力学和动力学稳定性之间存在很大偏差;这可能会对未来 BVMO 酶的发现及其合成应用产生重大影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号