当前位置:
X-MOL 学术
›
Nutr. Diabetes
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gpr17 deficiency in POMC neurons ameliorates the metabolic derangements caused by long-term high-fat diet feeding.
Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2019-10-14 , DOI: 10.1038/s41387-019-0096-7 Austin M Reilly 1 , Shudi Zhou 1 , Sunil K Panigrahi 2 , Shijun Yan 3, 4 , Jason M Conley 3, 4 , Patrick L Sheets 1, 5 , Sharon L Wardlaw 2 , Hongxia Ren 1, 3, 4, 5, 6, 7
Nutrition & Diabetes ( IF 4.6 ) Pub Date : 2019-10-14 , DOI: 10.1038/s41387-019-0096-7 Austin M Reilly 1 , Shudi Zhou 1 , Sunil K Panigrahi 2 , Shijun Yan 3, 4 , Jason M Conley 3, 4 , Patrick L Sheets 1, 5 , Sharon L Wardlaw 2 , Hongxia Ren 1, 3, 4, 5, 6, 7
Affiliation
|
BACKGROUND
Proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARH) control energy homeostasis by sensing hormonal and nutrient cues and activating secondary melanocortin sensing neurons. We identified the expression of a G protein-coupled receptor, Gpr17, in the ARH and hypothesized that it contributes to the regulatory function of POMC neurons on metabolism.
METHODS
In order to test this hypothesis, we generated POMC neuron-specific Gpr17 knockout (PGKO) mice and determined their energy and glucose metabolic phenotypes on normal chow diet (NCD) and high-fat diet (HFD).
RESULTS
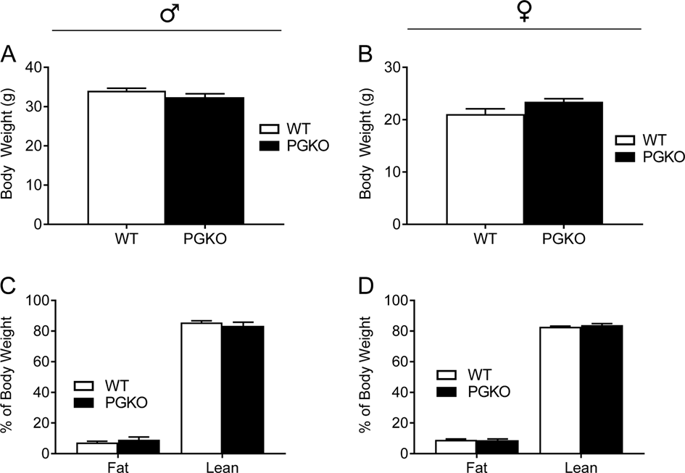
Adult PGKO mice on NCD displayed comparable body composition and metabolic features measured by indirect calorimetry. By contrast, PGKO mice on HFD demonstrated a sexually dimorphic phenotype with female PGKO mice displaying better metabolic homeostasis. Notably, female PGKO mice gained significantly less body weight and adiposity (p < 0.01), which was associated with increased energy expenditure, locomotor activity, and respiratory quotient, while males did not have an overt change in energy homeostasis. Though PGKO mice of both sexes had comparable glucose and insulin tolerance, detailed analyses of liver gene expression and serum metabolites indicate that PGKO mice could have reduced gluconeogenesis and increased lipid utilization on HFD. To elucidate the central-based mechanism(s) underlying the better-preserved energy and glucose homeostasis in PGKO mice on HFD, we examined the electrophysiological properties of POMC neurons and found Gpr17 deficiency led to increased spontaneous action potentials. Moreover, PGKO mice, especially female knockouts, had increased POMC-derived alpha-melanocyte stimulating hormone and beta-endorphin despite a comparable level of prohormone POMC in their hypothalamic extracts.
CONCLUSIONS
Gpr17 deficiency in POMC neurons protects metabolic homeostasis in a sex-dependent manner during dietary and aging challenges, suggesting that Gpr17 could be an effective anti-obesity target in specific populations with poor metabolic control.
中文翻译:

POMC神经元的Gpr17缺乏症改善了长期高脂饮食喂养引起的代谢紊乱。
背景技术下丘脑(ARH)弓形核中的Proopiomelanocortin(POMC)神经元通过感测激素和营养信号并激活次级黑皮质素感测神经元来控制能量稳态。我们确定了AR受体中G蛋白偶联受体Gpr17的表达,并假设它有助于POMC神经元对代谢的调节功能。方法为了验证这一假设,我们生成了POMC神经元特异性Gpr17基因敲除(PGKO)小鼠,并通过正常食物(NCD)和高脂饮食(HFD)确定了它们的能量和葡萄糖代谢表型。结果NCD上的成年PGKO小鼠显示出可比的身体组成和通过间接量热法测量的代谢特征。相比之下,HFD上的PGKO小鼠表现出性二态性表型,雌性PGKO小鼠表现出更好的代谢稳态。值得注意的是,雌性PGKO小鼠的体重和肥胖率显着降低(p <0.01),这与能量消耗,运动能力和呼吸商增加有关,而雄性则没有明显的能量稳态变化。尽管两种性别的PGKO小鼠均具有相当的葡萄糖和胰岛素耐受性,但对肝脏基因表达和血清代谢产物的详细分析表明,PGKO小鼠可以减少糖原异生并增加HFD的脂质利用。为了阐明HFKO PGKO小鼠中保存更好的能量和葡萄糖稳态的基础中心机制,我们检查了POMC神经元的电生理特性,发现Gpr17缺乏导致自发动作电位增加。此外,尽管PGKO小鼠,尤其是雌性敲除小鼠的下丘脑提取物中的激素原POMC水平相当,但其POMC衍生的α-黑素细胞刺激激素和β-内啡肽的含量却有所增加。结论在饮食和衰老挑战期间,POMC神经元的Gpr17缺乏以性别相关的方式保护了代谢稳态,这表明Gpr17可能是代谢控制不良的特定人群的有效抗肥胖目标。
更新日期:2019-10-14
中文翻译:

POMC神经元的Gpr17缺乏症改善了长期高脂饮食喂养引起的代谢紊乱。
背景技术下丘脑(ARH)弓形核中的Proopiomelanocortin(POMC)神经元通过感测激素和营养信号并激活次级黑皮质素感测神经元来控制能量稳态。我们确定了AR受体中G蛋白偶联受体Gpr17的表达,并假设它有助于POMC神经元对代谢的调节功能。方法为了验证这一假设,我们生成了POMC神经元特异性Gpr17基因敲除(PGKO)小鼠,并通过正常食物(NCD)和高脂饮食(HFD)确定了它们的能量和葡萄糖代谢表型。结果NCD上的成年PGKO小鼠显示出可比的身体组成和通过间接量热法测量的代谢特征。相比之下,HFD上的PGKO小鼠表现出性二态性表型,雌性PGKO小鼠表现出更好的代谢稳态。值得注意的是,雌性PGKO小鼠的体重和肥胖率显着降低(p <0.01),这与能量消耗,运动能力和呼吸商增加有关,而雄性则没有明显的能量稳态变化。尽管两种性别的PGKO小鼠均具有相当的葡萄糖和胰岛素耐受性,但对肝脏基因表达和血清代谢产物的详细分析表明,PGKO小鼠可以减少糖原异生并增加HFD的脂质利用。为了阐明HFKO PGKO小鼠中保存更好的能量和葡萄糖稳态的基础中心机制,我们检查了POMC神经元的电生理特性,发现Gpr17缺乏导致自发动作电位增加。此外,尽管PGKO小鼠,尤其是雌性敲除小鼠的下丘脑提取物中的激素原POMC水平相当,但其POMC衍生的α-黑素细胞刺激激素和β-内啡肽的含量却有所增加。结论在饮食和衰老挑战期间,POMC神经元的Gpr17缺乏以性别相关的方式保护了代谢稳态,这表明Gpr17可能是代谢控制不良的特定人群的有效抗肥胖目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号