npj Vaccines ( IF 6.9 ) Pub Date : 2019-10-14 , DOI: 10.1038/s41541-019-0135-3 Giada Mattiuzzo 1 , Ivana Knezevic 2 , Mark Hassall 1 , James Ashall 1 , Sophie Myhill 1 , Valwynne Faulkner 1 , Jason Hockley 3 , Peter Rigsby 3 , Dianna E Wilkinson 1 , Mark Page 1 ,
|
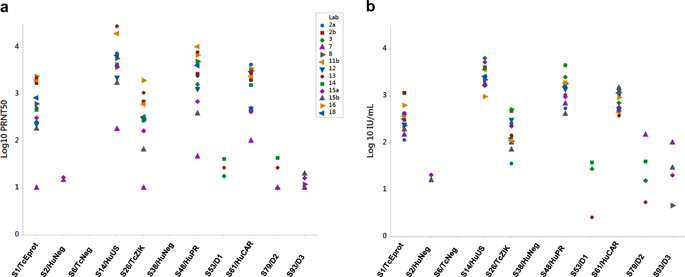
During outbreaks of emerging viruses, such as the Zika outbreak in 2015–2016, speed and accuracy in detection of infection are critical factors to control the spread of the disease; often serological and diagnostic methods for emerging viruses are not well developed and validated. Thus, vaccines and treatments are difficult to evaluate due to the lack of comparable methods. In this study, we show how the 1st WHO International Standard for anti-Zika antibody was able to harmonize the neutralization titres of a panel of serological Zika-positive samples from laboratories worldwide. Expression of the titres in International Unit per millilitre reduced the inter-laboratory variance, allowing for greater comparability between laboratories. We advocate the use of the International Standard for anti-Zika virus antibodies for the calibration of neutralization assays to create a common language, which will permit a clear evaluation of the results of different clinical trials and expedite the vaccine/treatment development.
中文翻译:

使用世界卫生组织抗寨卡病毒抗体国际标准协调寨卡中和测定
在新出现的病毒爆发期间,例如2015-2016年的寨卡病毒爆发,感染检测的速度和准确性是控制疾病传播的关键因素;通常,新兴病毒的血清学和诊断方法尚未得到充分开发和验证。因此,由于缺乏可比较的方法,疫苗和治疗方法很难评估。在这项研究中,我们展示了第一个世界卫生组织抗寨卡抗体国际标准如何能够协调来自世界各地实验室的一组寨卡病毒血清学阳性样本的中和滴度。以每毫升国际单位表示的滴度减少了实验室间的差异,从而提高了实验室之间的可比性。我们主张使用抗寨卡病毒抗体国际标准来校准中和测定,以创建通用语言,这将允许对不同临床试验的结果进行清晰的评估,并加快疫苗/治疗的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号