Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multisystem Proteinopathy Mutations in VCP/p97 Increase NPLOC4·UFD1L Binding and Substrate Processing.
Structure ( IF 5.7 ) Pub Date : 2019-10-14 , DOI: 10.1016/j.str.2019.09.011 Emily E Blythe 1 , Stephanie N Gates 2 , Raymond J Deshaies 3 , Andreas Martin 2
Structure ( IF 5.7 ) Pub Date : 2019-10-14 , DOI: 10.1016/j.str.2019.09.011 Emily E Blythe 1 , Stephanie N Gates 2 , Raymond J Deshaies 3 , Andreas Martin 2
Affiliation
|
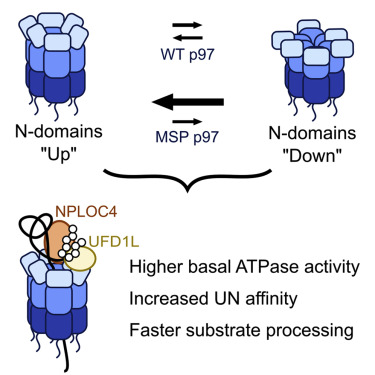
Valosin-containing protein (VCP)/p97 is an essential ATP-dependent protein unfoldase. Dominant mutations in p97 cause multisystem proteinopathy (MSP), a disease affecting the brain, muscle, and bone. Despite the identification of numerous pathways that are perturbed in MSP, the molecular-level defects of these p97 mutants are not completely understood. Here, we use biochemistry and cryoelectron microscopy to explore the effects of MSP mutations on the unfoldase activity of p97 in complex with its substrate adaptor NPLOC4⋅UFD1L (UN). We show that all seven analyzed MSP mutants unfold substrates faster. Mutant homo- and heterohexamers exhibit tighter UN binding and faster substrate processing. Our structural studies suggest that the increased UN affinity originates from a decoupling of p97's nucleotide state and the positioning of its N-terminal domains. Together, our data support a gain-of-function model for p97-UN-dependent processes in MSP and underscore the importance of N-terminal domain movements for adaptor recruitment and substrate processing by p97.
中文翻译:

VCP / p97中的多系统蛋白病突变增加NPLOC4·UFD1L结合和底物加工。
含Valosin的蛋白质(VCP)/ p97是必不可少的ATP依赖性蛋白质解折叠酶。p97中的显着突变会导致多系统蛋白病(MSP),这种疾病会影响大脑,肌肉和骨骼。尽管已鉴定出许多受MSP干扰的途径,但这些p97突变体的分子水平缺陷尚不完全清楚。在这里,我们使用生物化学和冷冻电子显微镜研究了MSP突变对其底物衔接子NPLOC4·UFD1L(UN)的复合物中p97的解折叠酶活性的影响。我们表明,所有七个分析的MSP突变体都能更快地展开底物。突变的同六聚体和异六聚体表现出更紧密的UN结合和更快的底物处理。我们的结构研究表明,联合国亲和力增加源自p97'的解偶联 核苷酸状态及其N末端结构域的位置。总之,我们的数据支持MSP中p97-UN依赖性过程的功能获得模型,并强调了p97适配器募集和底物处理过程中N末端域移动的重要性。
更新日期:2019-10-14
中文翻译:

VCP / p97中的多系统蛋白病突变增加NPLOC4·UFD1L结合和底物加工。
含Valosin的蛋白质(VCP)/ p97是必不可少的ATP依赖性蛋白质解折叠酶。p97中的显着突变会导致多系统蛋白病(MSP),这种疾病会影响大脑,肌肉和骨骼。尽管已鉴定出许多受MSP干扰的途径,但这些p97突变体的分子水平缺陷尚不完全清楚。在这里,我们使用生物化学和冷冻电子显微镜研究了MSP突变对其底物衔接子NPLOC4·UFD1L(UN)的复合物中p97的解折叠酶活性的影响。我们表明,所有七个分析的MSP突变体都能更快地展开底物。突变的同六聚体和异六聚体表现出更紧密的UN结合和更快的底物处理。我们的结构研究表明,联合国亲和力增加源自p97'的解偶联 核苷酸状态及其N末端结构域的位置。总之,我们的数据支持MSP中p97-UN依赖性过程的功能获得模型,并强调了p97适配器募集和底物处理过程中N末端域移动的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号