当前位置:
X-MOL 学术
›
Nat. Rev. Urol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The future of clinical trials in urological oncology.
Nature Reviews Urology ( IF 12.1 ) Pub Date : 2019-10-11 , DOI: 10.1038/s41585-019-0243-x Vikram M Narayan 1, 2 , Philipp Dahm 1
Nature Reviews Urology ( IF 12.1 ) Pub Date : 2019-10-11 , DOI: 10.1038/s41585-019-0243-x Vikram M Narayan 1, 2 , Philipp Dahm 1
Affiliation
|
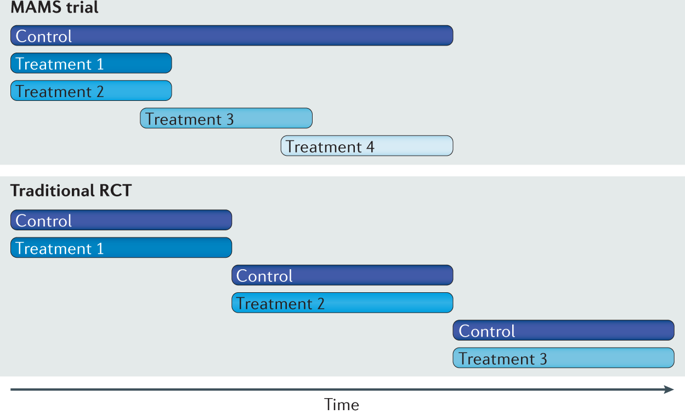
Well-designed clinical trials in urological oncology help to guide treatment decisions and aid in counselling patients, ultimately serving to improve outcomes. Since the term evidence-based medicine was first used by Gordon Guyatt in 1991, a renewed emphasis on methodology, transparent trial design and study reporting has helped to improve clinical research and in turn, the landscape of medical literature. Novel clinical trial designs (including multi-arm, multistage trials, basket and umbrella studies and research from big data sources, such as electronic health records, administrative claims databases and quality monitoring registries) are well suited to advance innovation in urological oncology. Existing urological clinical trials are often limited by small numbers, are statistically underpowered and many face difficulties with accrual. Thus, efforts to improve trial design are of considerable importance. The development and use of standard outcome sets and adherence to reporting guidelines offer researchers the opportunity to guide value-oriented care, minimize research waste and efficiently identify solutions to the unanswered questions in urology cancer care.
中文翻译:

泌尿肿瘤临床试验的未来。
泌尿肿瘤学中精心设计的临床试验有助于指导治疗决策并帮助为患者提供咨询,最终有助于改善结果。自 1991 年 Gordon Guyatt 首次使用循证医学一词以来,重新强调方法论、透明的试验设计和研究报告有助于改善临床研究,进而改善医学文献的前景。新的临床试验设计(包括多臂、多阶段试验、篮子和伞式研究以及来自大数据源的研究,如电子健康记录、行政索赔数据库和质量监测登记处)非常适合推进泌尿肿瘤学的创新。现有的泌尿外科临床试验通常受到少数人的限制,统计数据不足,并且许多人面临应计困难。因此,改进试验设计的努力非常重要。标准结果集的开发和使用以及对报告指南的遵守为研究人员提供了指导以价值为导向的护理、最大限度地减少研究浪费并有效地确定泌尿科癌症护理中未解决问题的解决方案的机会。
更新日期:2019-10-12
中文翻译:

泌尿肿瘤临床试验的未来。
泌尿肿瘤学中精心设计的临床试验有助于指导治疗决策并帮助为患者提供咨询,最终有助于改善结果。自 1991 年 Gordon Guyatt 首次使用循证医学一词以来,重新强调方法论、透明的试验设计和研究报告有助于改善临床研究,进而改善医学文献的前景。新的临床试验设计(包括多臂、多阶段试验、篮子和伞式研究以及来自大数据源的研究,如电子健康记录、行政索赔数据库和质量监测登记处)非常适合推进泌尿肿瘤学的创新。现有的泌尿外科临床试验通常受到少数人的限制,统计数据不足,并且许多人面临应计困难。因此,改进试验设计的努力非常重要。标准结果集的开发和使用以及对报告指南的遵守为研究人员提供了指导以价值为导向的护理、最大限度地减少研究浪费并有效地确定泌尿科癌症护理中未解决问题的解决方案的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号