The Lancet HIV ( IF 12.8 ) Pub Date : 2019-10-11 , DOI: 10.1016/s2352-3018(19)30338-8 Takara L Stanley 1 , Lindsay T Fourman 1 , Meghan N Feldpausch 1 , Julia Purdy 2 , Isabel Zheng 1 , Chelsea S Pan 1 , Julia Aepfelbacher 2 , Colleen Buckless 3 , Andrew Tsao 3 , Anela Kellogg 4 , Karen Branch 5 , Hang Lee 6 , Chia-Ying Liu 7 , Kathleen E Corey 8 , Raymond T Chung 8 , Martin Torriani 3 , David E Kleiner 9 , Colleen M Hadigan 2 , Steven K Grinspoon 1
|
Background
Non-alcoholic fatty liver disease (NAFLD) is a substantial cause of comorbidity in people with HIV and there are no proven pharmacological treatments for the disease in this population. We assessed the effects of tesamorelin on liver fat and histology in people with HIV and NAFLD.
Methods
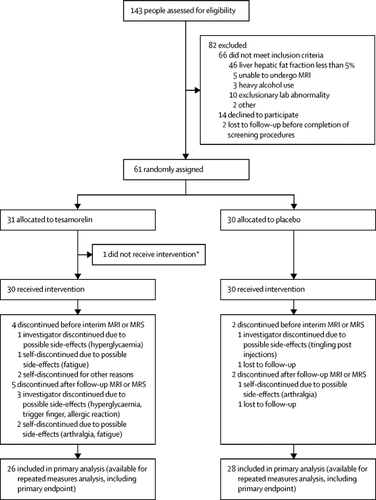
This randomised, double-blind, multicentre study with identical placebo as a comparator was done in a hospital and a medical research centre in the USA. People with HIV infection and a hepatic fat fraction (HFF) of 5% or more by proton magnetic resonance spectroscopy were eligible. Participants were randomly assigned (1:1) to receive either tesamorelin 2 mg once daily or placebo once daily for 12 months, followed by a 6-month open-label phase during which all participants received tesamorelin 2 mg daily. The randomisation list was prepared by the study statistician using a permuted block algorithm within each stratum with randomly varying block sizes. The primary endpoint was change in HFF between baseline and 12 months. The primary safety endpoint was glucose. Analysis was by intention to treat using all available data. This trial is registered with ClinicalTrials.gov, number NCT02196831.
Findings
61 patients were enrolled between Aug 20, 2015, and Jan 16, 2019, of whom 30 received tesamorelin and 30 received placebo. Patients receiving tesamorelin had a greater reduction of HFF than did patients receiving placebo, with an absolute effect size of −4·1% (95% CI −7·6 to −0·7, p=0·018), corresponding to a −37% (95% CI −67 to −7, p=0·016) relative reduction from baseline. After 12 months, 35% of individuals receiving tesamorelin and 4% receiving placebo had a HFF of less than 5% (p=0·0069). Changes in fasting glucose and glycated haemoglobin were not different between groups at 12 months. Individuals in the tesamorelin group experienced more localised injection site complaints than those in the placebo group, though none were judged to be serious.
Interpretation
Tesamorelin might be beneficial in people with HIV and NAFLD. Further studies are needed to determine the long-term effects of tesamorelin on liver histology.
Funding
National Institutes of Health and National Institute of Allergy and Infectious Diseases.
中文翻译:

替沙莫林对艾滋病毒非酒精性脂肪肝的影响:一项随机、双盲、多中心试验。
背景
非酒精性脂肪肝 (NAFLD) 是 HIV 感染者合并症的一个重要原因,目前尚无针对该人群的有效药物治疗方法。我们评估了替沙莫林对 HIV 和 NAFLD 患者肝脏脂肪和组织学的影响。
方法
这项随机、双盲、多中心研究在美国的一家医院和一个医学研究中心进行,以相同的安慰剂作为对照。患有 HIV 感染且质子磁共振波谱检测肝脂肪分数 (HFF) 为 5% 或以上的人符合资格。参与者被随机分配 (1:1) 接受 tesamorelin 2 mg 每日一次或安慰剂每日一次,持续 12 个月,然后是为期 6 个月的开放标签阶段,在此期间所有参与者每天接受 tesamorelin 2 mg。随机化列表是由研究统计学家在每个层中使用置换块算法来准备的,块大小随机变化。主要终点是基线和 12 个月之间 HFF 的变化。主要安全终点是葡萄糖。分析是有意使用所有可用数据进行治疗。该试验已在 ClinicalTrials.gov 注册,编号为 NCT02196831。
发现
2015年8月20日至2019年1月16日期间入组了61名患者,其中30人接受替沙莫林治疗,30人接受安慰剂治疗。与接受安慰剂的患者相比,接受替沙莫林的患者 HFF 降低幅度更大,绝对效应大小为 -4·1%(95% CI -7·6 至 -0·7,p=0·018),相当于-37% (95% CI -67 至 -7, p=0·016) 相对于基线减少。 12 个月后,接受 tesamorelin 治疗的个体中有 35% 接受安慰剂治疗的个体有 4% 的 HFF 低于 5% (p=0·0069)。 12 个月时,各组之间的空腹血糖和糖化血红蛋白的变化没有差异。与安慰剂组相比,替沙莫林组的个体经历了更多的局部注射部位不适,但没有一个被认为是严重的。
解释
替沙莫林可能对 HIV 和 NAFLD 患者有益。需要进一步的研究来确定替沙莫林对肝脏组织学的长期影响。
资金
美国国立卫生研究院和国家过敏和传染病研究所。











































 京公网安备 11010802027423号
京公网安备 11010802027423号