当前位置:
X-MOL 学术
›
Nanoscale Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Remote-controlled multi-enzyme system for enhanced tumor therapy via dark/light relay catalysis
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2019/10/11 , DOI: 10.1039/c9nh00583h Ying Chen 1, 2, 3, 4 , Zi-Hao Li 1, 2, 3, 4 , Jing-Jing Hu 1, 2, 3, 4 , Si-Yuan Peng 1, 2, 3, 4 , Lei Rong 1, 2, 3, 4 , Yunxia Sun 1, 2, 3, 4 , Xian-Zheng Zhang 1, 2, 3, 4
Nanoscale Horizons ( IF 8.0 ) Pub Date : 2019/10/11 , DOI: 10.1039/c9nh00583h Ying Chen 1, 2, 3, 4 , Zi-Hao Li 1, 2, 3, 4 , Jing-Jing Hu 1, 2, 3, 4 , Si-Yuan Peng 1, 2, 3, 4 , Lei Rong 1, 2, 3, 4 , Yunxia Sun 1, 2, 3, 4 , Xian-Zheng Zhang 1, 2, 3, 4
Affiliation
|
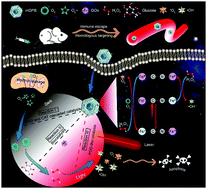
Nanozymes have been widely used in biomedicine, especially in tumor therapy. However, inadequate H2O2 supply in the tumor microenvironment (TME) and uncontrolled catalytic activity of nanozymes in vivo restrict their practical application. Here, a dark/light relay strategy is proposed to realize adequate H2O2 supply in the dark reaction stage and selectively perform desired catalytic activities for toxic reactive oxygen species (ROS) generation to kill tumors by remote light control. A tumor membrane camouflaged and glucose oxidase (GOx) loaded hollow mesoporous Prussian blue (mGPB) nanosystem is designed to target tumor tissues for homologous aggregation of membranes. The cascaded catalysis of superoxide dismutase (SOD) and catalase (CAT)-like activities inherited from hollow mesoporous Prussian blue (HMPB) efficiently catalyze endogenous O2−˙ to O2, which contributes to the oxidative decomposition of glucose to produce H2O2 by loaded GOx. Moreover, mGPB nanoparticles are found to utilize H2O2 to produce ˙OH and 1O2 under NIR irradiation via other light-dependent dual-catalytic properties, acting as peroxidase (POD) and oxidase (OXD). By dark/light relay catalysis, we successfully overcome the limited H2O2 supply in TME and achieve precise ROS generation, displaying prominent tumor suppression in mouse xenograft models.
中文翻译:

遥控多酶系统,通过暗/亮中继催化增强肿瘤治疗
纳米酶已被广泛用于生物医学,特别是在肿瘤治疗中。然而,肿瘤微环境(TME)中H 2 O 2的供应不足以及纳米酶在体内的不受控制的催化活性限制了它们的实际应用。在此,提出了一种暗/亮中继策略以实现足够的H 2 O 2。在黑暗的反应阶段提供能量,并选择性地执行所需的催化活性,以产生有毒的活性氧(ROS),从而通过远程光控制杀死肿瘤。肿瘤膜伪装和葡萄糖氧化酶(GOx)加载空心中孔普鲁士蓝(mGPB)纳米系统旨在针对肿瘤组织的膜的同源聚集。空心中孔普鲁士蓝(HMPB)继承的超氧化物歧化酶(SOD)和过氧化氢酶(CAT)类活性的级联催化有效地将内源性O 2 - ˙催化为O 2,这有助于葡萄糖氧化分解产生H 2 O 2由加载的GOx组成。此外,发现mGPB纳米颗粒利用H 2 O2在NIR辐照下,通过其他依赖光的双重催化性质,生成˙OH和1 O 2,起过氧化物酶(POD)和氧化酶(OXD)的作用。通过暗/光中继催化,我们成功克服了TME中有限的H 2 O 2供给并实现了精确的ROS生成,在小鼠异种移植模型中显示出显着的肿瘤抑制作用。
更新日期:2020-02-13
中文翻译:

遥控多酶系统,通过暗/亮中继催化增强肿瘤治疗
纳米酶已被广泛用于生物医学,特别是在肿瘤治疗中。然而,肿瘤微环境(TME)中H 2 O 2的供应不足以及纳米酶在体内的不受控制的催化活性限制了它们的实际应用。在此,提出了一种暗/亮中继策略以实现足够的H 2 O 2。在黑暗的反应阶段提供能量,并选择性地执行所需的催化活性,以产生有毒的活性氧(ROS),从而通过远程光控制杀死肿瘤。肿瘤膜伪装和葡萄糖氧化酶(GOx)加载空心中孔普鲁士蓝(mGPB)纳米系统旨在针对肿瘤组织的膜的同源聚集。空心中孔普鲁士蓝(HMPB)继承的超氧化物歧化酶(SOD)和过氧化氢酶(CAT)类活性的级联催化有效地将内源性O 2 - ˙催化为O 2,这有助于葡萄糖氧化分解产生H 2 O 2由加载的GOx组成。此外,发现mGPB纳米颗粒利用H 2 O2在NIR辐照下,通过其他依赖光的双重催化性质,生成˙OH和1 O 2,起过氧化物酶(POD)和氧化酶(OXD)的作用。通过暗/光中继催化,我们成功克服了TME中有限的H 2 O 2供给并实现了精确的ROS生成,在小鼠异种移植模型中显示出显着的肿瘤抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号