Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
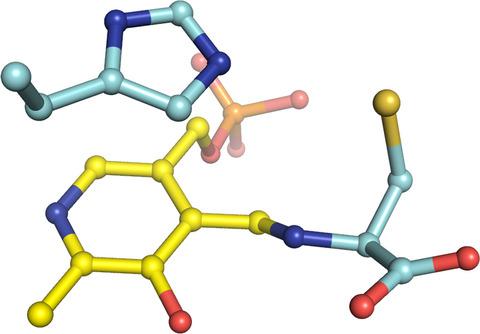
Snapshots of PLP-substrate and PLP-product external aldimines as intermediates in two types of cysteine desulfurase enzymes.
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-10-06 , DOI: 10.1111/febs.15081 Ryosuke Nakamura 1 , Masahide Hikita 2 , Shoko Ogawa 1 , Yasuhiro Takahashi 1 , Takashi Fujishiro 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-10-06 , DOI: 10.1111/febs.15081 Ryosuke Nakamura 1 , Masahide Hikita 2 , Shoko Ogawa 1 , Yasuhiro Takahashi 1 , Takashi Fujishiro 1
Affiliation
|
Cysteine desulfurase enzymes catalyze sulfur mobilization from l-cysteine to sulfur-containing biomolecules such as iron-sulfur (Fe-S) clusters and thio-tRNAs. The enzymes utilize the cofactor pyridoxal-5'-phosphate (PLP), which forms the external substrate- and product-aldimines and ketimines during catalysis and are grouped into two types (I and II) based on their different catalytic loops. To clarify the structure-based catalytic mechanisms for each group, we determined the structures of the external substrate- and product-aldimines as catalytic intermediates of NifS (type I) and SufS (type II) that are involved in Fe-S cluster biosynthesis using X-ray crystallographic snapshot analysis. As a common intermediate structure, the thiol group of the PLP-l-cysteine external aldimine is stabilized by the conserved histidine adjacent to PLP through a polar interaction. This interaction makes the thiol group orientated for subsequent nucleophilic attack by a conserved cysteine residue on the catalytic loop in the state of PLP-l-cysteine ketimine, which is formed from the PLP-l-cysteine aldimine. Unlike the intermediates, structural changes of the loops were different between the type I and II enzymes. In the type I enzyme, conformational and topological change of the loop is necessary for nucleophilic attack by the cysteine. In contrast, the loop in type II cysteine desulfurase enzymes showed no large conformational change; rather, it might possibly orient the thiol group of the catalytic cysteine for nucleophilic attack toward PLP-l-cysteine. The present structures allow a revision of the catalytic mechanism and may provide a clue for consideration of enzyme function, structural diversity, and evolution of cysteine desulfurase enzymes. DATABASE: Structural data are available in PDB database under the accession numbers 5WT2, 5WT4, 5ZSP, 5ZST, 5ZS9, 5ZSK, 5ZSO, 6KFZ, 6KG0, and 6KG1.
中文翻译:

作为两种半胱氨酸脱硫酶中间产物的PLP底物和PLP产物外部亚胺的快照。
半胱氨酸脱硫酶催化硫从L-半胱氨酸转移到含硫生物分子,如铁硫(Fe-S)簇和硫代tRNA。这些酶利用了辅因子5'-磷酸吡phosphate醛(PLP),该酶在催化过程中形成了外部底物和产物醛亚胺和酮亚胺,并根据其不同的催化环分为两种类型(I和II)。为了阐明每个组的基于结构的催化机制,我们确定了外部底物和产物醛亚胺的结构,这些结构是NifS(I型)和SufS(II型)的催化中间体,参与了Fe-S团簇的生物合成。 X射线晶体学快照分析。作为常见的中间结构,PLP-1-半胱氨酸外部亚胺的硫醇基团通过极性相互作用被与PLP相邻的保守组氨酸稳定。这种相互作用使硫醇基团定向成随后由PLP-1-半胱氨酸酮亚胺形成的PLP-1-半胱氨酸酮亚胺状态的催化环上的保守半胱氨酸残基随后的亲核攻击。与中间体不同,环的结构变化在I型和II型酶之间不同。在I型酶中,环的构象和拓扑变化对于半胱氨酸的亲核攻击是必需的。相比之下,II型半胱氨酸脱硫酶中的环没有显示出大的构象变化。相反,它可能使催化性半胱氨酸的硫醇基团定向为对PLP-1-半胱氨酸的亲核攻击。本结构允许修改催化机理,并且可以为考虑酶功能,结构多样性和半胱氨酸脱硫酶的进化提供线索。数据库:结构数据可在PDB数据库中获得,登录号为5WT2、5WT4、5ZSP,5ZST,5ZS9、5ZSK,5ZSO,6KFZ,6KG0和6KG1。
更新日期:2020-03-16
中文翻译:

作为两种半胱氨酸脱硫酶中间产物的PLP底物和PLP产物外部亚胺的快照。
半胱氨酸脱硫酶催化硫从L-半胱氨酸转移到含硫生物分子,如铁硫(Fe-S)簇和硫代tRNA。这些酶利用了辅因子5'-磷酸吡phosphate醛(PLP),该酶在催化过程中形成了外部底物和产物醛亚胺和酮亚胺,并根据其不同的催化环分为两种类型(I和II)。为了阐明每个组的基于结构的催化机制,我们确定了外部底物和产物醛亚胺的结构,这些结构是NifS(I型)和SufS(II型)的催化中间体,参与了Fe-S团簇的生物合成。 X射线晶体学快照分析。作为常见的中间结构,PLP-1-半胱氨酸外部亚胺的硫醇基团通过极性相互作用被与PLP相邻的保守组氨酸稳定。这种相互作用使硫醇基团定向成随后由PLP-1-半胱氨酸酮亚胺形成的PLP-1-半胱氨酸酮亚胺状态的催化环上的保守半胱氨酸残基随后的亲核攻击。与中间体不同,环的结构变化在I型和II型酶之间不同。在I型酶中,环的构象和拓扑变化对于半胱氨酸的亲核攻击是必需的。相比之下,II型半胱氨酸脱硫酶中的环没有显示出大的构象变化。相反,它可能使催化性半胱氨酸的硫醇基团定向为对PLP-1-半胱氨酸的亲核攻击。本结构允许修改催化机理,并且可以为考虑酶功能,结构多样性和半胱氨酸脱硫酶的进化提供线索。数据库:结构数据可在PDB数据库中获得,登录号为5WT2、5WT4、5ZSP,5ZST,5ZS9、5ZSK,5ZSO,6KFZ,6KG0和6KG1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号