当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of cation redox chemistry on continuous hydrothermal synthesis of 2D-Ni(Co/Fe) hydroxides
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-10-08 , DOI: 10.1039/c9re00334g Massimo Rosa 1, 2, 3, 4 , Debora Marani 5, 6, 7, 8, 9 , Giovanni Perin 10, 11, 12, 13 , Søren Bredmose Simonsen 1, 2, 3, 4 , Philipp Zielke 1, 2, 3, 4 , Antonella Glisenti 10, 11, 12, 13 , Ragnar Kiebach 1, 2, 3, 4 , Andreas Lesch 14, 15, 16, 17 , Vincenzo Esposito 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2019-10-08 , DOI: 10.1039/c9re00334g Massimo Rosa 1, 2, 3, 4 , Debora Marani 5, 6, 7, 8, 9 , Giovanni Perin 10, 11, 12, 13 , Søren Bredmose Simonsen 1, 2, 3, 4 , Philipp Zielke 1, 2, 3, 4 , Antonella Glisenti 10, 11, 12, 13 , Ragnar Kiebach 1, 2, 3, 4 , Andreas Lesch 14, 15, 16, 17 , Vincenzo Esposito 1, 2, 3, 4
Affiliation
|
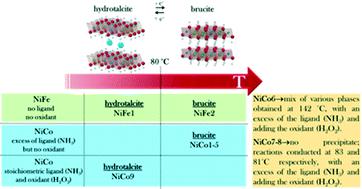
Continuous hydrothermal flow synthesis (CHFS) is a facile, upscalable and cost-efficient synthetic method enabling the nanostructuring of advanced functional materials in steady conditions, i.e. not in batch synthesis. In this paper, we use CHFS to crystallize NiCo- and NiFe-hydroxides in water solution with 2D nanofeatures. By tuning the synthetic parameters, we disclose the key role of the cation redox chemistry in the transition between two competitive phases: from 2D-nanoplatelets of brucite to layered double hydroxides (LDH). For controlling the precipitation of different Ni, Fe, Co-hydroxide phases, we propose the combined use of an oxidizing (H2O2) and a complexing (NH3) agent. At temperatures as low as 80 °C, the presence of H2O2 and a low concentration of NH3 favour the Ni2+/Co3+ over Ni2+/Co2+ oxidation states, shifting the product structure from brucite phase (temperatures > 80 °C) to LDH. Conversely, for the NiFe-hydroxides the transition from LDH (temperatures ≤ 80 °C) to brucite phase (temperatures > 80 °C) is controlled by the reaction temperature only. Due to the high stability of Fe3+, the synthesis of NiFe products by CHFS does not require oxidizing and complexing agents, resulting in a robust process for large-scale production.
中文翻译:

阳离子氧化还原化学对2D-Ni(Co / Fe)氢氧化物连续水热合成的影响
连续水热流合成(CHFS)是一种简便,可升级且具有成本效益的合成方法,能够在稳定条件下(即不在分批合成中)对先进功能材料进行纳米结构化。在本文中,我们使用CHFS在具有二维纳米特征的水溶液中结晶NiCo-和NiFe-氢氧化物。通过调整合成参数,我们揭示了阳离子氧化还原化学在两个竞争阶段之间的过渡中的关键作用:从水镁石的二维纳米片晶到层状双氢氧化物(LDH)。为了控制不同的Ni,Fe,Co-氢氧化物相的沉淀,我们建议将氧化(H 2 O 2)和络合物(NH 3) 代理人。在低至80°C的温度下,H 2 O 2的存在和低浓度的NH 3比Ni 2+ / Co 2+的氧化态更有利于Ni 2+ / Co 3+的发生,从而使产品结构从水镁石相转变(温度> 80°C)至LDH。相反,对于氢氧化镍铁,从LDH(温度≤80°C)到水镁石相(温度> 80°C)的转变仅受反应温度控制。由于Fe 3+的高稳定性,通过CHFS合成NiFe产物不需要氧化剂和络合剂,因此可实现大规模生产的可靠过程。
更新日期:2019-10-08
中文翻译:

阳离子氧化还原化学对2D-Ni(Co / Fe)氢氧化物连续水热合成的影响
连续水热流合成(CHFS)是一种简便,可升级且具有成本效益的合成方法,能够在稳定条件下(即不在分批合成中)对先进功能材料进行纳米结构化。在本文中,我们使用CHFS在具有二维纳米特征的水溶液中结晶NiCo-和NiFe-氢氧化物。通过调整合成参数,我们揭示了阳离子氧化还原化学在两个竞争阶段之间的过渡中的关键作用:从水镁石的二维纳米片晶到层状双氢氧化物(LDH)。为了控制不同的Ni,Fe,Co-氢氧化物相的沉淀,我们建议将氧化(H 2 O 2)和络合物(NH 3) 代理人。在低至80°C的温度下,H 2 O 2的存在和低浓度的NH 3比Ni 2+ / Co 2+的氧化态更有利于Ni 2+ / Co 3+的发生,从而使产品结构从水镁石相转变(温度> 80°C)至LDH。相反,对于氢氧化镍铁,从LDH(温度≤80°C)到水镁石相(温度> 80°C)的转变仅受反应温度控制。由于Fe 3+的高稳定性,通过CHFS合成NiFe产物不需要氧化剂和络合剂,因此可实现大规模生产的可靠过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号