当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of fused heterocyclic systems via the Mallory photoreaction of arylthienylethenes.
Photochemical & Photobiological Sciences ( IF 3.1 ) Pub Date : 2019-10-28 , DOI: 10.1039/c9pp00289h Natalia V Dyachenko 1 , Andrey V Khoroshutin 2 , Yulia A Sotnikova 2 , Valentina A Karnoukhova 3 , Sergey D Tokarev 1 , Alexander V Anisimov 2 , Yurii V Fedorov 3 , Olga A Fedorova 1
Photochemical & Photobiological Sciences ( IF 3.1 ) Pub Date : 2019-10-28 , DOI: 10.1039/c9pp00289h Natalia V Dyachenko 1 , Andrey V Khoroshutin 2 , Yulia A Sotnikova 2 , Valentina A Karnoukhova 3 , Sergey D Tokarev 1 , Alexander V Anisimov 2 , Yurii V Fedorov 3 , Olga A Fedorova 1
Affiliation
|
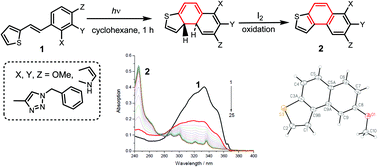
Photochemical oxidative cyclization of 2- and 3-thienylstilbenes (heterostilbenes) containing mono-, di- and trimethoxy groups in the benzene ring or heterocyclic fragment results in the formation of isomeric thieno-annelated polycyclic aromatic compounds demonstrating optical properties that differ from those of initial stilbene derivatives. The structures of cyclic products were evaluated via1H and 13C NMR, HRMS, elemental analysis and X-ray crystallography. The research was aimed to study the effect of substituents in stilbene derivatives of thiophene as well as the position of the styryl fragment in the thiophene nucleus on the occurrence of photocyclization reactions.
中文翻译:

通过芳基噻吩基乙烯的马洛里光反应合成稠合杂环系统。
苯环或杂环片段中含有单,二和三甲氧基的2-和3-噻吩基苯乙烯(杂苯)的光化学氧化环化导致形成异噻吩基退火的多环芳族化合物,这表明其光学性质不同于最初的光学性质二苯乙烯衍生物。通过1 H和13 C NMR,HRMS,元素分析和X射线晶体学对环状产物的结构进行了评估。该研究旨在研究噻吩的二苯乙烯衍生物中的取代基以及噻吩核中苯乙烯基片段的位置对光环化反应发生的影响。
更新日期:2019-12-04
中文翻译:

通过芳基噻吩基乙烯的马洛里光反应合成稠合杂环系统。
苯环或杂环片段中含有单,二和三甲氧基的2-和3-噻吩基苯乙烯(杂苯)的光化学氧化环化导致形成异噻吩基退火的多环芳族化合物,这表明其光学性质不同于最初的光学性质二苯乙烯衍生物。通过1 H和13 C NMR,HRMS,元素分析和X射线晶体学对环状产物的结构进行了评估。该研究旨在研究噻吩的二苯乙烯衍生物中的取代基以及噻吩核中苯乙烯基片段的位置对光环化反应发生的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号