Nature Metabolism ( IF 20.8 ) Pub Date : 2019-10-07 , DOI: 10.1038/s42255-019-0120-1 Riikka H Hämäläinen 1, 2 , Juan C Landoni 2 , Kati J Ahlqvist 2 , Steffi Goffart 3 , Sanna Ryytty 1 , M Obaidur Rahman 1 , Virginia Brilhante 2 , Katherine Icay 4 , Sampsa Hautaniemi 4 , Liya Wang 5 , Marikki Laiho 6 , Anu Suomalainen 2, 7, 8
|
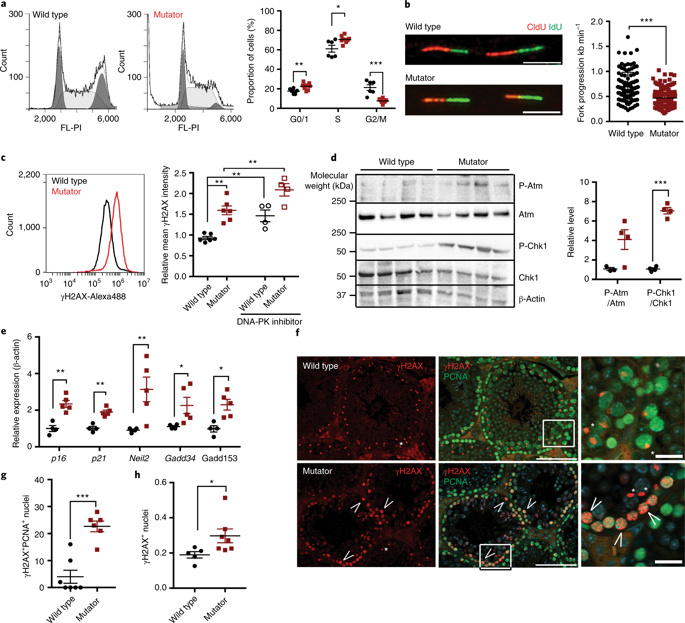
Mitochondrial DNA (mtDNA) mutagenesis and nuclear DNA repair defects are considered cellular mechanisms of ageing. mtDNA mutator mice with increased mtDNA mutagenesis show signs of premature ageing. However, why patients with mitochondrial diseases, or mice with other forms of mitochondrial dysfunction, do not age prematurely remains unknown. Here, we show that cells from mutator mice display challenged nuclear genome maintenance similar to that observed in progeric cells with defects in nuclear DNA repair. Cells from mutator mice show slow nuclear DNA replication fork progression, cell cycle stalling and chronic DNA replication stress, leading to double-strand DNA breaks in proliferating progenitor or stem cells. The underlying mechanism involves increased mtDNA replication frequency, sequestering of nucleotides to mitochondria, depletion of total cellular nucleotide pools, decreased deoxynucleoside 5′-triphosphate (dNTP) availability for nuclear genome replication and compromised nuclear genome maintenance. Our data indicate that defects in mtDNA replication can challenge nuclear genome stability. We suggest that defects in nuclear genome maintenance, particularly in the stem cell compartment, represent a unified mechanism for mouse progerias. Therefore, through their destabilizing effects on the nuclear genome, mtDNA mutations are indirect contributors to organismal ageing, suggesting that the direct role of mtDNA mutations in driving ageing-like symptoms might need to be revisited.
中文翻译:

mtDNA复制中的缺陷通过核苷酸耗竭挑战了核基因组的稳定性,并为小鼠早衰症提供了统一的机制。
线粒体DNA(mtDNA)诱变和核DNA修复缺陷被认为是细胞衰老的机制。mtDNA诱变增加的mtDNA突变小鼠表现出过早衰老的迹象。但是,为什么线粒体疾病患者或其他形式的线粒体功能障碍的小鼠为何不早衰仍然未知。在这里,我们显示来自突变小鼠的细胞显示出挑战性的核基因组维护,类似于在具有核DNA修复缺陷的早老细胞中观察到的维护。突变小鼠的细胞显示出缓慢的核DNA复制叉进展,细胞周期停滞和长期的DNA复制压力,导致增殖的祖细胞或干细胞中的双链DNA断裂。潜在的机制包括增加mtDNA复制频率,将核苷酸隔离到线粒体,总细胞核苷酸池的耗竭,核基因组复制的脱氧核苷5'-三磷酸(dNTP)可用性降低和核基因组维护受损。我们的数据表明,mtDNA复制中的缺陷可以挑战核基因组的稳定性。我们建议核基因组维护中的缺陷,特别是在干细胞区室中的缺陷,代表了小鼠早衰的统一机制。因此,通过其对核基因组的破坏作用,mtDNA突变是机体衰老的间接原因,这表明可能需要重新研究mtDNA突变在驱动衰老样症状中的直接作用。我们的数据表明,mtDNA复制中的缺陷可以挑战核基因组的稳定性。我们建议核基因组维护中的缺陷,特别是在干细胞区室中的缺陷,代表了小鼠早衰的统一机制。因此,通过其对核基因组的破坏作用,mtDNA突变是机体衰老的间接原因,这表明可能需要重新研究mtDNA突变在驱动衰老样症状中的直接作用。我们的数据表明,mtDNA复制中的缺陷可以挑战核基因组的稳定性。我们建议核基因组维护中的缺陷,特别是在干细胞区室中的缺陷,代表了小鼠早衰的统一机制。因此,通过其对核基因组的破坏作用,mtDNA突变是机体衰老的间接原因,这表明可能需要重新研究mtDNA突变在驱动衰老样症状中的直接作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号