Nature Catalysis ( IF 42.8 ) Pub Date : 2019-10-07 , DOI: 10.1038/s41929-019-0357-9 Yanjun Li , Meng Lei , Lei Gong
|
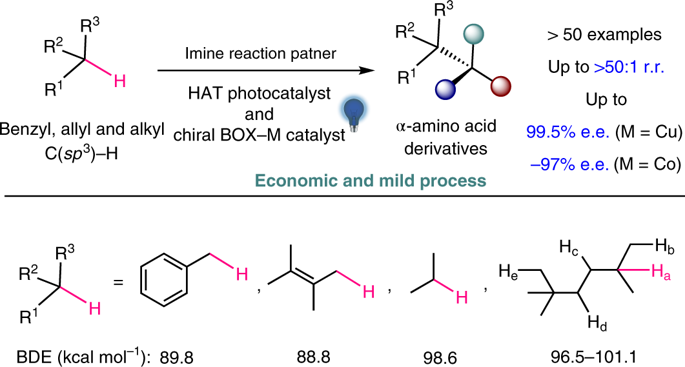
The selective functionalization of inert C(sp3)–H bonds is extremely attractive in organic synthesis and catalysis science, but the conversion of hydrocarbons lacking directing groups into chiral molecules through catalytic C(sp3)–H functionalization is formidably challenging. Here, to address this problem, we have developed a photochemical system consisting of a hydrogen atom transfer organophotocatalyst and a chiral catalyst containing an earth-abundant metal. With the cooperative catalysts and imine partners, a wide range of benzylic, allylic hydrocarbons and unactivated alkanes can be converted to functionalized chiral products. The readily tunable bisoxazoline catalysts of copper or other metals exhibit precise regional recognition and asymmetric induction towards these inert C–H bonds. The reactions are applicable to many compounds including small hydrocarbons, branched alkanes, cycloalkanes and more complex medicinal agents. This method provides an economic and rapid construction of optically active compounds, starting from the most basic chemical feedstocks.
中文翻译:

苄基和烯丙基烃以及未活化烷烃的光催化区域和立体选择性C(sp 3)–H功能化
惰性C(sp 3)–H键的选择性功能化在有机合成和催化科学中极具吸引力,但缺乏通过催化C(sp 3)引导基团转化为手性分子的碳氢化合物)– H功能强大的挑战。在这里,为了解决这个问题,我们已经开发了一种光化学体系,该体系由氢原子转移有机光催化剂和一种含有富地球金属的手性催化剂组成。使用协同催化剂和亚胺伙伴,可以将各种苄基,烯丙基烃和未活化的烷烃转化为官能化的手性产物。铜或其他金属的易于调节的双恶唑啉催化剂表现出精确的区域识别性,并且对这些惰性C–H键具有不对称诱导作用。该反应适用于许多化合物,包括小烃,支链烷烃,环烷烃和更复杂的药物。从最基本的化学原料开始,该方法可经济,快速地构建旋光化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号