Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-10-04 , DOI: 10.1016/s0140-6736(19)32222-6 Luis Paz-Ares 1 , Mikhail Dvorkin 2 , Yuanbin Chen 3 , Niels Reinmuth 4 , Katsuyuki Hotta 5 , Dmytro Trukhin 6 , Galina Statsenko 7 , Maximilian J Hochmair 8 , Mustafa Özgüroğlu 9 , Jun Ho Ji 10 , Oleksandr Voitko 11 , Artem Poltoratskiy 12 , Santiago Ponce 1 , Francesco Verderame 13 , Libor Havel 14 , Igor Bondarenko 15 , Andrzej Kazarnowicz 16 , György Losonczy 17 , Nikolay V Conev 18 , Jon Armstrong 19 , Natalie Byrne 19 , Norah Shire 20 , Haiyi Jiang 20 , Jonathan W Goldman 21 ,
The Lancet ( IF 98.4 ) Pub Date : 2019-10-04 , DOI: 10.1016/s0140-6736(19)32222-6 Luis Paz-Ares 1 , Mikhail Dvorkin 2 , Yuanbin Chen 3 , Niels Reinmuth 4 , Katsuyuki Hotta 5 , Dmytro Trukhin 6 , Galina Statsenko 7 , Maximilian J Hochmair 8 , Mustafa Özgüroğlu 9 , Jun Ho Ji 10 , Oleksandr Voitko 11 , Artem Poltoratskiy 12 , Santiago Ponce 1 , Francesco Verderame 13 , Libor Havel 14 , Igor Bondarenko 15 , Andrzej Kazarnowicz 16 , György Losonczy 17 , Nikolay V Conev 18 , Jon Armstrong 19 , Natalie Byrne 19 , Norah Shire 20 , Haiyi Jiang 20 , Jonathan W Goldman 21 ,
Affiliation
|
BACKGROUND
Most patients with small-cell lung cancer (SCLC) have extensive-stage disease at presentation, and prognosis remains poor. Recently, immunotherapy has demonstrated clinical activity in extensive-stage SCLC (ES-SCLC). The CASPIAN trial assessed durvalumab, with or without tremelimumab, in combination with etoposide plus either cisplatin or carboplatin (platinum-etoposide) in treatment-naive patients with ES-SCLC.
METHODS
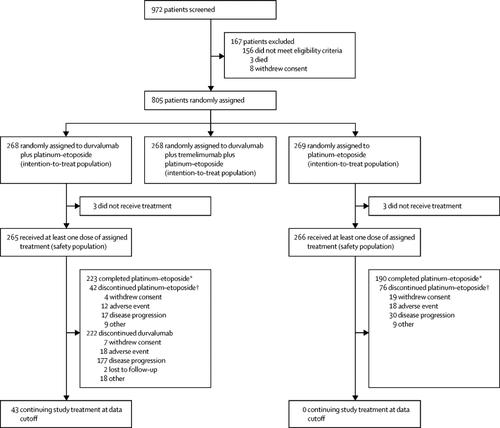
This randomised, open-label, phase 3 trial was done at 209 sites across 23 countries. Eligible patients were adults with untreated ES-SCLC, with WHO performance status 0 or 1 and measurable disease as per Response Evaluation Criteria in Solid Tumors, version 1.1. Patients were randomly assigned (in a 1:1:1 ratio) to durvalumab plus platinum-etoposide; durvalumab plus tremelimumab plus platinum-etoposide; or platinum-etoposide alone. All drugs were administered intravenously. Platinum-etoposide consisted of etoposide 80-100 mg/m2 on days 1-3 of each cycle with investigator's choice of either carboplatin area under the curve 5-6 mg/mL per min or cisplatin 75-80 mg/m2 (administered on day 1 of each cycle). Patients received up to four cycles of platinum-etoposide plus durvalumab 1500 mg with or without tremelimumab 75 mg every 3 weeks followed by maintenance durvalumab 1500 mg every 4 weeks in the immunotherapy groups and up to six cycles of platinum-etoposide every 3 weeks plus prophylactic cranial irradiation (investigator's discretion) in the platinum-etoposide group. The primary endpoint was overall survival in the intention-to-treat population. We report results for the durvalumab plus platinum-etoposide group versus the platinum-etoposide group from a planned interim analysis. Safety was assessed in all patients who received at least one dose of their assigned study treatment. This study is registered at ClinicalTrials.gov, NCT03043872, and is ongoing.
FINDINGS
Patients were enrolled between March 27, 2017, and May 29, 2018. 268 patients were allocated to the durvalumab plus platinum-etoposide group and 269 to the platinum-etoposide group. Durvalumab plus platinum-etoposide was associated with a significant improvement in overall survival, with a hazard ratio of 0·73 (95% CI 0·59-0·91; p=0·0047]); median overall survival was 13·0 months (95% CI 11·5-14·8) in the durvalumab plus platinum-etoposide group versus 10·3 months (9·3-11·2) in the platinum-etoposide group, with 34% (26·9-41·0) versus 25% (18·4-31·6) of patients alive at 18 months. Any-cause adverse events of grade 3 or 4 occurred in 163 (62%) of 265 treated patients in the durvalumab plus platinum-etoposide group and 166 (62%) of 266 in the platinum-etoposide group; adverse events leading to death occurred in 13 (5%) and 15 (6%) patients.
INTERPRETATION
First-line durvalumab plus platinum-etoposide significantly improved overall survival in patients with ES-SCLC versus a clinically relevant control group. Safety findings were consistent with the known safety profiles of all drugs received.
FUNDING
AstraZeneca.
中文翻译:

在广泛期小细胞肺癌(CASPIAN)一线治疗中,Durvalumab联合铂-依托泊苷与铂-依托泊苷的比较:一项随机,对照,开放标签的3期临床试验。
背景技术大多数小细胞肺癌(SCLC)患者在病情呈广泛阶段时,预后仍然很差。最近,免疫疗法已在广泛阶段的SCLC(ES-SCLC)中证明了临床活性。CASPIAN试验评估了初治ES-SCLC患者的durvalumab(含或不含tremelimumab)与依托泊苷联合顺铂或卡铂(铂-依托泊苷)的组合。方法这项随机,开放标签的3期临床试验在23个国家/地区的209个地点进行。符合条件的患者是成人,未经治疗的ES-SCLC,WHO表现状态为0或1,并且根据《实体肿瘤缓解评估标准》(版本1.1)可测量疾病。患者被随机分配(以1:1:1的比例)地鲁比单抗加铂-依托泊苷;durvalumab加上tremelimumab加上铂-依托泊苷;或单独使用铂-依托泊苷。所有药物均静脉内给药。铂-依托泊苷由每个周期的第1-3天的依托泊苷80-100 mg / m2组成,研究人员选择曲线下每分钟5-6 mg / mL的卡铂面积或顺铂75-80 mg / m2(每天给药)每个周期1个)。在免疫治疗组中,患者每3周接受多达四个周期的铂-依托泊苷加1500 mg的durvalumab联合或不联合tremelimumab 75 mg,随后在免疫治疗组中每4周接受维持durvalumab 1500 mg的联合治疗(每3周)多达六个周期的铂-依托泊苷联合预防性治疗铂-依托泊苷组进行颅骨照射(研究者酌情决定)。主要终点是意图治疗人群的总体生存率。我们从计划的中期分析报告了durvalumab联合铂-依托泊苷组与铂-依托泊苷组的结果。在接受至少一剂指定研究治疗剂量的所有患者中评估安全性。该研究已在ClinicalTrials.gov上注册,编号为NCT03043872,目前正在进行中。结果在2017年3月27日至2018年5月29日之间招募了患者。将268例患者分配给durvalumab加铂-依托泊苷组,将269例分配给铂-依托泊苷组。Durvalumab联合铂-依托泊苷可显着改善总体生存率,危险比为0·73(95%CI 0·59-0·91; p = 0·0047])。durvalumab联合铂-依托泊苷组的中位总生存期为13·0个月(95%CI 11·5-14·8),而铂-依托泊苷组为10·3个月(9·3-11·2),在18个月时存活的患者为34%(26·9-41·0),而25%(18·4-31·6)的患者存活。在接受durvalumab联合铂-依托泊苷治疗的265名患者中,有163名(62%)发生了3级或4级的任何原因的不良事件,在铂-依托泊苷组的266名患者中有166名(62%)发生了这种情况;导致死亡的不良事件发生在13(5%)和15(6%)患者中。解释与临床相关对照组相比,一线durvalumab联合铂-依托泊苷可显着改善ES-SCLC患者的总生存期。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。在接受durvalumab联合铂-依托泊苷治疗的265名患者中,有163名(62%)发生了3级或4级的任何原因的不良事件,在铂-依托泊苷组的266名患者中有166名(62%)发生了这种情况;导致死亡的不良事件发生在13(5%)和15(6%)患者中。解释与临床相关对照组相比,一线durvalumab联合铂-依托泊苷可显着改善ES-SCLC患者的总生存期。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。在接受durvalumab联合铂-依托泊苷治疗的265名患者中,有163名(62%)发生了3级或4级的任何原因的不良事件,在铂-依托泊苷组的266名患者中有166名(62%)发生了这种情况;导致死亡的不良事件发生在13(5%)和15(6%)患者中。解释与临床相关对照组相比,一线durvalumab联合铂-依托泊苷可显着改善ES-SCLC患者的总生存期。安全性发现与所收到的所有药物的已知安全性特征相符。资助阿斯利康。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。
更新日期:2019-11-22
中文翻译:

在广泛期小细胞肺癌(CASPIAN)一线治疗中,Durvalumab联合铂-依托泊苷与铂-依托泊苷的比较:一项随机,对照,开放标签的3期临床试验。
背景技术大多数小细胞肺癌(SCLC)患者在病情呈广泛阶段时,预后仍然很差。最近,免疫疗法已在广泛阶段的SCLC(ES-SCLC)中证明了临床活性。CASPIAN试验评估了初治ES-SCLC患者的durvalumab(含或不含tremelimumab)与依托泊苷联合顺铂或卡铂(铂-依托泊苷)的组合。方法这项随机,开放标签的3期临床试验在23个国家/地区的209个地点进行。符合条件的患者是成人,未经治疗的ES-SCLC,WHO表现状态为0或1,并且根据《实体肿瘤缓解评估标准》(版本1.1)可测量疾病。患者被随机分配(以1:1:1的比例)地鲁比单抗加铂-依托泊苷;durvalumab加上tremelimumab加上铂-依托泊苷;或单独使用铂-依托泊苷。所有药物均静脉内给药。铂-依托泊苷由每个周期的第1-3天的依托泊苷80-100 mg / m2组成,研究人员选择曲线下每分钟5-6 mg / mL的卡铂面积或顺铂75-80 mg / m2(每天给药)每个周期1个)。在免疫治疗组中,患者每3周接受多达四个周期的铂-依托泊苷加1500 mg的durvalumab联合或不联合tremelimumab 75 mg,随后在免疫治疗组中每4周接受维持durvalumab 1500 mg的联合治疗(每3周)多达六个周期的铂-依托泊苷联合预防性治疗铂-依托泊苷组进行颅骨照射(研究者酌情决定)。主要终点是意图治疗人群的总体生存率。我们从计划的中期分析报告了durvalumab联合铂-依托泊苷组与铂-依托泊苷组的结果。在接受至少一剂指定研究治疗剂量的所有患者中评估安全性。该研究已在ClinicalTrials.gov上注册,编号为NCT03043872,目前正在进行中。结果在2017年3月27日至2018年5月29日之间招募了患者。将268例患者分配给durvalumab加铂-依托泊苷组,将269例分配给铂-依托泊苷组。Durvalumab联合铂-依托泊苷可显着改善总体生存率,危险比为0·73(95%CI 0·59-0·91; p = 0·0047])。durvalumab联合铂-依托泊苷组的中位总生存期为13·0个月(95%CI 11·5-14·8),而铂-依托泊苷组为10·3个月(9·3-11·2),在18个月时存活的患者为34%(26·9-41·0),而25%(18·4-31·6)的患者存活。在接受durvalumab联合铂-依托泊苷治疗的265名患者中,有163名(62%)发生了3级或4级的任何原因的不良事件,在铂-依托泊苷组的266名患者中有166名(62%)发生了这种情况;导致死亡的不良事件发生在13(5%)和15(6%)患者中。解释与临床相关对照组相比,一线durvalumab联合铂-依托泊苷可显着改善ES-SCLC患者的总生存期。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。在接受durvalumab联合铂-依托泊苷治疗的265名患者中,有163名(62%)发生了3级或4级的任何原因的不良事件,在铂-依托泊苷组的266名患者中有166名(62%)发生了这种情况;导致死亡的不良事件发生在13(5%)和15(6%)患者中。解释与临床相关对照组相比,一线durvalumab联合铂-依托泊苷可显着改善ES-SCLC患者的总生存期。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。在接受durvalumab联合铂-依托泊苷治疗的265名患者中,有163名(62%)发生了3级或4级的任何原因的不良事件,在铂-依托泊苷组的266名患者中有166名(62%)发生了这种情况;导致死亡的不良事件发生在13(5%)和15(6%)患者中。解释与临床相关对照组相比,一线durvalumab联合铂-依托泊苷可显着改善ES-SCLC患者的总生存期。安全性发现与所收到的所有药物的已知安全性特征相符。资助阿斯利康。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。安全性研究结果与所收到的所有药物的已知安全性特征一致。资助阿斯利康。











































 京公网安备 11010802027423号
京公网安备 11010802027423号