Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Apicomplexan F-actin is required for efficient nuclear entry during host cell invasion.
EMBO Reports ( IF 6.5 ) Pub Date : 2019-10-04 , DOI: 10.15252/embr.201948896 Mario Del Rosario 1 , Javier Periz 1 , Georgios Pavlou 2 , Oliver Lyth 3 , Fernanda Latorre-Barragan 1, 4 , Sujaan Das 1 , Gurman S Pall 1 , Johannes Felix Stortz 1 , Leandro Lemgruber 1 , Jamie A Whitelaw 5 , Jake Baum 3 , Isabelle Tardieux 2 , Markus Meissner 1, 6
EMBO Reports ( IF 6.5 ) Pub Date : 2019-10-04 , DOI: 10.15252/embr.201948896 Mario Del Rosario 1 , Javier Periz 1 , Georgios Pavlou 2 , Oliver Lyth 3 , Fernanda Latorre-Barragan 1, 4 , Sujaan Das 1 , Gurman S Pall 1 , Johannes Felix Stortz 1 , Leandro Lemgruber 1 , Jamie A Whitelaw 5 , Jake Baum 3 , Isabelle Tardieux 2 , Markus Meissner 1, 6
Affiliation
|
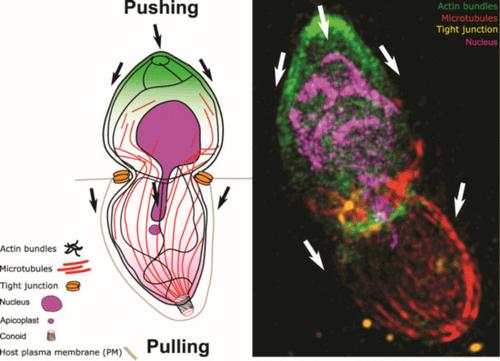
The obligate intracellular parasites Toxoplasma gondii and Plasmodium spp. invade host cells by injecting a protein complex into the membrane of the targeted cell that bridges the two cells through the assembly of a ring-like junction. This circular junction stretches while the parasites apply a traction force to pass through, a step that typically concurs with transient constriction of the parasite body. Here we analyse F-actin dynamics during host cell invasion. Super-resolution microscopy and real-time imaging highlighted an F-actin pool at the apex of pre-invading parasite, an F-actin ring at the junction area during invasion but also networks of perinuclear and posteriorly localised F-actin. Mutant parasites with dysfunctional acto-myosin showed significant decrease of junctional and perinuclear F-actin and are coincidently affected in nuclear passage through the junction. We propose that the F-actin machinery eases nuclear passage by stabilising the junction and pushing the nucleus through the constriction. Our analysis suggests that the junction opposes resistance to the passage of the parasite's nucleus and provides the first evidence for a dual contribution of actin-forces during host cell invasion by apicomplexan parasites.
中文翻译:

顶复体 F-肌动蛋白是宿主细胞侵袭过程中有效进入核所必需的。
专性细胞内寄生虫弓形虫和疟原虫属。通过将蛋白质复合物注射到目标细胞的膜中,通过环状连接的组装将两个细胞桥接起来,从而侵入宿主细胞。当寄生虫施加牵引力穿过该圆形连接处时,该圆形连接处会伸展,这一步骤通常与寄生虫身体的短暂收缩同时发生。在这里,我们分析了宿主细胞侵袭过程中的 F-肌动蛋白动态。超分辨率显微镜和实时成像突出显示了入侵前寄生虫顶端的 F-肌动蛋白池、入侵过程中交界处的 F-肌动蛋白环以及核周和后部定位的 F-肌动蛋白网络。具有功能障碍的肌动球蛋白的突变寄生虫表现出交界处和核周F-肌动蛋白的显着减少,并且在通过交界处的核通道中同时受到影响。我们提出 F-肌动蛋白机制通过稳定连接并推动细胞核通过收缩来缓解核通过。我们的分析表明,该连接反对寄生虫细胞核通过的阻力,并为顶复门寄生虫入侵宿主细胞期间肌动蛋白力的双重贡献提供了第一个证据。
更新日期:2019-12-05
中文翻译:

顶复体 F-肌动蛋白是宿主细胞侵袭过程中有效进入核所必需的。
专性细胞内寄生虫弓形虫和疟原虫属。通过将蛋白质复合物注射到目标细胞的膜中,通过环状连接的组装将两个细胞桥接起来,从而侵入宿主细胞。当寄生虫施加牵引力穿过该圆形连接处时,该圆形连接处会伸展,这一步骤通常与寄生虫身体的短暂收缩同时发生。在这里,我们分析了宿主细胞侵袭过程中的 F-肌动蛋白动态。超分辨率显微镜和实时成像突出显示了入侵前寄生虫顶端的 F-肌动蛋白池、入侵过程中交界处的 F-肌动蛋白环以及核周和后部定位的 F-肌动蛋白网络。具有功能障碍的肌动球蛋白的突变寄生虫表现出交界处和核周F-肌动蛋白的显着减少,并且在通过交界处的核通道中同时受到影响。我们提出 F-肌动蛋白机制通过稳定连接并推动细胞核通过收缩来缓解核通过。我们的分析表明,该连接反对寄生虫细胞核通过的阻力,并为顶复门寄生虫入侵宿主细胞期间肌动蛋白力的双重贡献提供了第一个证据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号