Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis of Paxillin Recruitment by Kindlin-2 in Regulating Cell Adhesion.
Structure ( IF 4.4 ) Pub Date : 2019-10-04 , DOI: 10.1016/j.str.2019.09.006 Liang Zhu 1 , Huan Liu 2 , Fan Lu 3 , Jun Yang 1 , Tatiana V Byzova 2 , Jun Qin 3
Structure ( IF 4.4 ) Pub Date : 2019-10-04 , DOI: 10.1016/j.str.2019.09.006 Liang Zhu 1 , Huan Liu 2 , Fan Lu 3 , Jun Yang 1 , Tatiana V Byzova 2 , Jun Qin 3
Affiliation
|
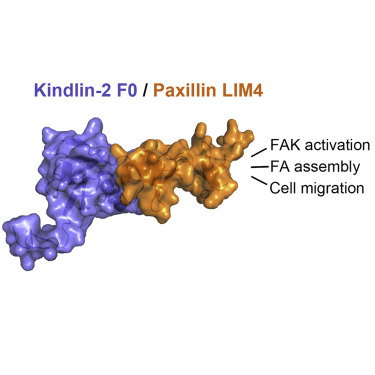
Activation of cell surface receptor integrin has been extensively studied as the first key step to trigger cell adhesion, but the subsequent events, widely regarded as integrin "outside-in" signaling to form supramolecular complexes (focal adhesions [FAs]) to promote dynamic cell adhesion, remain poorly elucidated. Integrin activator kindlin-2 was recently found to associate with paxillin in nascent FAs, implicating an early yet undefined integrin outside-in signaling event. Here we show structurally that kindlin-2 recognizes paxillin via a distinct interface involving the ubiquitin-like kindlin-2 F0 domain and the paxillin LIM4 domain. The interface is adjacent to the membrane binding site of kindlin-2 F0, suggesting a mechanism for kindlin-2 to recruit paxillin to the membrane-proximal site where FA assembly is initiated. Disruption of the interface impaired the localization of paxillin, causing strong defects in FA assembly and cell migration. These data unveil a structural basis of the kindlin-2/paxillin interaction in controlling dynamic cell adhesion.
中文翻译:

Kindlin-2 在调节细胞粘附中招募桩蛋白的结构基础。
细胞表面受体整合素的激活作为触发细胞粘附的第一个关键步骤已被广泛研究,但随后的事件被广泛认为是整合素“由外向内”信号传导形成超分子复合物(粘着斑[FA])以促进细胞动态附着力,目前尚不清楚。最近发现整合素激活剂 kindlin-2 与新生 FA 中的桩蛋白相关,这意味着早期但未定义的整合素由外向内信号传导事件。在这里,我们从结构上证明 kindlin-2 通过涉及泛素样 kindlin-2 F0 结构域和桩蛋白 LIM4 结构域的独特界面识别桩蛋白。该界面与 kindlin-2 F0 的膜结合位点相邻,表明 kindlin-2 存在一种将桩蛋白招募到 FA 组装启动的近膜位点的机制。界面的破坏损害了桩蛋白的定位,导致 FA 组装和细胞迁移出现严重缺陷。这些数据揭示了 kindlin-2/paxillin 相互作用在控制动态细胞粘附中的结构基础。
更新日期:2019-10-04
中文翻译:

Kindlin-2 在调节细胞粘附中招募桩蛋白的结构基础。
细胞表面受体整合素的激活作为触发细胞粘附的第一个关键步骤已被广泛研究,但随后的事件被广泛认为是整合素“由外向内”信号传导形成超分子复合物(粘着斑[FA])以促进细胞动态附着力,目前尚不清楚。最近发现整合素激活剂 kindlin-2 与新生 FA 中的桩蛋白相关,这意味着早期但未定义的整合素由外向内信号传导事件。在这里,我们从结构上证明 kindlin-2 通过涉及泛素样 kindlin-2 F0 结构域和桩蛋白 LIM4 结构域的独特界面识别桩蛋白。该界面与 kindlin-2 F0 的膜结合位点相邻,表明 kindlin-2 存在一种将桩蛋白招募到 FA 组装启动的近膜位点的机制。界面的破坏损害了桩蛋白的定位,导致 FA 组装和细胞迁移出现严重缺陷。这些数据揭示了 kindlin-2/paxillin 相互作用在控制动态细胞粘附中的结构基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号