当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-solvating, side-chain polymer electrolytes as lithium single-ion conductors: synthesis and ion transport characterization†
Polymer Chemistry ( IF 4.1 ) Pub Date : 2019-10-03 , DOI: 10.1039/c9py01035a Jiacheng Liu 1, 2, 3, 4 , Phillip D. Pickett 4, 5, 6, 7, 8 , Bumjun Park 1, 2, 3, 4 , Sunil P. Upadhyay 1, 2, 3, 4 , Sara V. Orski 4, 5, 6, 7, 8 , Jennifer L. Schaefer 1, 2, 3, 4
Polymer Chemistry ( IF 4.1 ) Pub Date : 2019-10-03 , DOI: 10.1039/c9py01035a Jiacheng Liu 1, 2, 3, 4 , Phillip D. Pickett 4, 5, 6, 7, 8 , Bumjun Park 1, 2, 3, 4 , Sunil P. Upadhyay 1, 2, 3, 4 , Sara V. Orski 4, 5, 6, 7, 8 , Jennifer L. Schaefer 1, 2, 3, 4
Affiliation
|
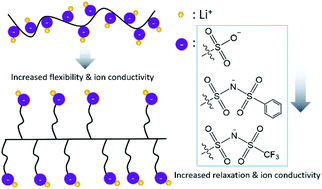
Solid-state single-ion conducting polymer electrolytes have drawn considerable interest for secondary lithium batteries due to their potential for high electrochemical stability and safety, but applications are limited by their low ionic conductivities. Specifically, poly(ethylene oxide) (PEO) based electrolytes have the highest reported Li+ conductivities for these materials; however, their potential is limited due to the ion transport mechanism being coupled to segmental relaxations of the cation solvating polymer chain. To investigate the potential of single-ion conducting polymer electrolytes lacking polar matrices, we synthesized three para-polyphenylene-based, side-chain polymer electrolytes with various pendent anion chemistries (–SO3−, –PSI−, and –TFSI−) with differing binding affinities to Li+. Compared with the previously reported lithium poly(4-styrenesulfonyl(trifluoromethylsulfonyl)imide) (LiPSTFSI), the side-chain polymers showed at least 3 orders of magnitude higher conductivity with the same –TFSI− anion (6.7 × 10−6 S cm−1 compared with 1.2 × 10−10 S cm−1 at 150 °C). We found that the side-chain electrolyte showed a dielectric relaxation dominated transport mechanism through use of dielectric spectroscopy analysis. The conductivity is highly dependent on the charge delocalization and size of the pendent anion, which provides a pathway forward for the engineering of polymeric ion conductors for electrochemical applications.
中文翻译:

非溶剂型侧链聚合物电解质作为锂单离子导体:合成和离子迁移表征†
固态单离子导电聚合物电解质由于其具有高电化学稳定性和安全性的潜力而引起了二次锂电池的极大兴趣,但是其低离子电导率限制了其应用。具体地说,聚环氧乙烷(PEO)基电解质对这些材料的Li +电导率最高。然而,由于离子传输机制与阳离子溶剂化聚合物链的节段松弛偶联,因此它们的潜力受到限制。为了研究的单离子导电聚合物电解质缺乏极性基质的潜力,我们合成了三种对基于-polyphenylene-,侧链的聚合物电解质与各种阴离子侧化学(-SO 3 -,–PSI −和–TFSI −)具有与Li +不同的结合亲和力。与先前报道的锂聚(4- styrenesulfonyl(三氟甲基磺酰)亚胺)(LiPSTFSI)相比,侧链聚合物显示出具有相同的数量级-TFSI更高电导率中的至少3个数量级-阴离子(6.7×10 -6小号厘米- 1为1.2×10 -10 S cm -1在150°C时)。我们发现,通过使用介电谱分析,侧链电解质显示出介电弛豫占主导的传输机制。电导率高度依赖于电荷的离域和悬垂阴离子的大小,这为工程化用于电化学应用的聚合物离子导体提供了一条前进的途径。
更新日期:2020-01-02
中文翻译:

非溶剂型侧链聚合物电解质作为锂单离子导体:合成和离子迁移表征†
固态单离子导电聚合物电解质由于其具有高电化学稳定性和安全性的潜力而引起了二次锂电池的极大兴趣,但是其低离子电导率限制了其应用。具体地说,聚环氧乙烷(PEO)基电解质对这些材料的Li +电导率最高。然而,由于离子传输机制与阳离子溶剂化聚合物链的节段松弛偶联,因此它们的潜力受到限制。为了研究的单离子导电聚合物电解质缺乏极性基质的潜力,我们合成了三种对基于-polyphenylene-,侧链的聚合物电解质与各种阴离子侧化学(-SO 3 -,–PSI −和–TFSI −)具有与Li +不同的结合亲和力。与先前报道的锂聚(4- styrenesulfonyl(三氟甲基磺酰)亚胺)(LiPSTFSI)相比,侧链聚合物显示出具有相同的数量级-TFSI更高电导率中的至少3个数量级-阴离子(6.7×10 -6小号厘米- 1为1.2×10 -10 S cm -1在150°C时)。我们发现,通过使用介电谱分析,侧链电解质显示出介电弛豫占主导的传输机制。电导率高度依赖于电荷的离域和悬垂阴离子的大小,这为工程化用于电化学应用的聚合物离子导体提供了一条前进的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号